Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nakagawa, Hiroshi; Jochi, Yasumasa*; Kitao, Akio*; Yamamuro, Osamu*; Kataoka, Mikio*
Biophysical Journal, 117(2), p.229 - 238, 2019/07
Times Cited Count:3 Percentile:13.03(Biophysics)Softness and rigidity of proteins are reflected in the structural dynamics, which are in turn affected by the environment. The characteristic low-frequency vibrational spectrum of a protein, known as boson peak, is an indication of the structural rigidity of the protein at cryogenic temperature or dehydrated conditions. In this paper, the effect of hydration, temperature, and pressure on the boson peak and volumetric properties of a globular protein are evaluated by using inelastic neutron scattering and molecular dynamics simulation. Hydration, pressurization, and cooling shift the boson peak position to higher energy and depress the peak intensity and decreases the protein and cavity volumes, although pressure hardly affects the boson peak of the fully hydrated protein. A decrease of each volume means the increase of rigidity, which is the origin of the boson peak shift. The boson peak profile can be predicted by the total cavity volume. This prediction is effective for the evaluation of the net quasielastic scattering of incoherent neutron scattering spectra when the boson peak cannot be distinguished experimentally because of a strong contribution from quasielastic scattering.
Yang, L.-W.*; Kitao, Akio*; Huang, B.-C.*; Go, Nobuhiro*
Biophysical Journal, 107(6), p.1415 - 1425, 2014/09
Times Cited Count:18 Percentile:54.3(Biophysics)Higuchi, Mariko; Fujii, Jumpei*; Yonetani, Yoshiteru; Kitao, Akio*; Go, Nobuhiro*
Journal of Structural Biology, 173(1), p.20 - 28, 2011/01
Times Cited Count:2 Percentile:6.15(Biochemistry & Molecular Biology)MutT distinguishes substrate 8-oxo-dGTP from dGTP and also 8-oxo-dGMP from dGMP despite small differences of chemical structures between them. In this paper we show by the method of molecular dynamics simulation that the transition between conformational substates of MutT is a key mechanism for a high resolution molecular recognition of the differences between the very similar chemical compounds. The native state MutT has two conformational substates with similar free energies, each characterized by either open or close of two loops surrounding the substrate binding active site. Between the two substates, the open substate is more stable in free MutT and in dGMP-MutT complex, and the closed substate is more stable in 8-oxo-dGMP-MutT complex. A hydrogen bond between H7 atom of 8-oxo-dGMP and the sidechain of Asn119 plays a crucial role for maintaining the closed substate in 8-oxo-dGMP-MutT complex.
Nakagawa, Hiroshi; Jochi, Yasumasa*; Kitao, Akio*; Kataoka, Mikio
Biophysical Journal, 95(6), p.2916 - 2923, 2008/09
Times Cited Count:49 Percentile:78.26(Biophysics)Jochi, Yasumasa*; Nakagawa, Hiroshi; Kataoka, Mikio; Kitao, Akio*
Biophysical Journal, 94(11), p.4435 - 4443, 2008/06
Times Cited Count:24 Percentile:53.62(Biophysics)Hydration effects on protein dynamics were investigated by comparing the frequency dependence of the calculated neutron scattering spectra between full and minimal hydration states at temperatures between 100 and 300 K. The protein boson peak is observed in the frequency range 1-4 meV at 100 K in both states. The peak frequency in the minimal hydration state shifts to lower than that in the full hydration state. Protein motions with frequency higher than 4 meV were shown to undergo almost harmonic motion in both states at all temperatures simulated, whereas those with frequency lower than 1 meV dominate the total fluctuations above 220 K and contribute to the origin of the glass-like transition. At 300 K, the boson peak becomes buried in the quasi-elastic contributions in the full hydration state, but is still observed in the minimal hydration state. The boson peak is observed when protein dynamics are trapped within a local minimum of its energy surface. Protein motions, which contribute to the boson peak, are distributed throughout the whole protein. Fine structure of the dynamics structure factor is expected to be detected by the experiment if a high resolution instrument is developed in the near future.
Jochi, Yasumasa*; Nakagawa, Hiroshi; Kataoka, Mikio; Kitao, Akio*
Journal of Physical Chemistry B, 112(11), p.3522 - 3528, 2008/03
Times Cited Count:10 Percentile:25.44(Chemistry, Physical)Molecular dynamics simulations of crystalline Staphylococcal nuclease in full and minimal hydration states were performed to study hydration effects on protein dynamics at temperatures ranging from 100 to 300 K. In a full hydration state (hydration ratio in weight, h = 0.49), gaps are fully filled with water molecules, whereas only crystal waters are included in a minimal hydration state (h = 0.09). The inflection of the atomic mean-square fluctuation of protein as a function of temperature, known as the glass-like transition, is observed at 220 K in both cases, which is more significant in the full hydration state. By examining the temperature dependence of residual fluctuation, we found that the increase of fluctuations in the loop and terminal regions, which are exposed to water, is much greater than in other regions in the full hydration state, but the mobility of the corresponding regions are relatively restricted in the minimal hydration state by inter-molecular contact. The atomic mean-square fluctuation of water molecules in the full hydration state at 300 K is one order of magnitude greater than that in the minimal hydration state. Above the transition temperature, most water molecules in the full hydration state behave like bulk water, and act as a lubricant for protein dynamics. In contrast, water molecules in the minimal hydration state tend to form more hydrogen bonds with the protein, restricting the fluctuation of these water molecules to the level of the protein. Thus, inter-molecular interaction and solvent mobility are important to understand the glass-like transition in proteins.
Tokuhisa, Atsushi; Jochi, Yasumasa*; Nakagawa, Hiroshi; Kitao, Akio*; Kataoka, Mikio
Physical Review E, 75(4), p.041912_1 - 041912_8, 2007/05
Times Cited Count:20 Percentile:67.12(Physics, Fluids & Plasmas)Elastic incoherent neutron scattering (EINS) data can be approximated with a Gaussian function of q in a low q region. However, in a higher q region the deviation from a Gaussian function becomes non-negligible. Protein dynamic properties can be derived from the analyses of the non-Gaussian behavior, which has been experimentally investigated. To evaluate the origins of the non-Gaussian behavior of protein dynamics, we conducted a molecular dynamics (MD) simulation of Staphylococcal nuclease. Instead of the ordinary cumulant expansion, we decomposed the non-Gaussian terms into three components: (1) the component originating from the heterogeneity of the mean-square fluctuation, (2) that from the anisotropy, and (3) that from higher order terms such as anharmonicity. The MD simulation revealed various dynamics for each atom. The atomic motions are classified into three types: (1) "harmonic", (2) "anisotropic", and (3) "anharmonic". However, each atom has a different degree of anisotropy. The contribution of the anisotropy to the total scattering function averages out due to these differences. Anharmonic motion is described as the jump among multiple minima. The jump distance and the probability of the residence at one site vary from atom to atom. Each anharmonic component oscillates between positive and negative values. Thus, the contribution of the anharmonicity to the total scattering is canceled due to the variations in the anharmonicity. Consequently, the non-Gaussian behavior of the total EINS from a protein can be analyzed by the dynamical heterogeneity.
Ishida, Hisashi; Higuchi, Mariko; Yonetani, Yoshiteru*; Kano, Takuma; Jochi, Yasumasa*; Kitao, Akio*; Go, Nobuhiro
Annual Report of the Earth Simulator Center April 2005 - March 2006, p.237 - 240, 2007/01
no abstracts in English
Nakagawa, Hiroshi; Tokuhisa, Atsushi*; Kamikubo, Hironari*; Jochi, Yasumasa*; Kitao, Akio*; Kataoka, Mikio*
Materials Science & Engineering A, 442(1-2), p.356 - 360, 2006/12
Times Cited Count:4 Percentile:34.16(Nanoscience & Nanotechnology)The dynamical heterogeneity of a globular soluble protein was studied by elastic incoherent neutron scattering and molecular simulations. The q-dependence of the elastic incoherent neutron scattering shows a non-Gaussianity, a deviation from Gaussian approximation. We determined that the dynamical heterogeneity explains the non-Gaussianity, although the anharmonicity is also plausible origin. Molecular dynamics simulations confirmed that the non-Gaussianity is mainly due to the dynamical heterogeneity at a lower energy resolution, 
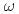 =1meV. On the other hand, the contribution from the anharmonicities to the non-Gaussianity became substantial at a higher resolution,
=1meV. On the other hand, the contribution from the anharmonicities to the non-Gaussianity became substantial at a higher resolution, 
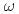 =10
=10 eV. Regardless, the dynamical heterogeneity is the dominant factor for the non-Gaussianity.
eV. Regardless, the dynamical heterogeneity is the dominant factor for the non-Gaussianity.
Nakagawa, Hiroshi; Kataoka, Mikio*; Jochi, Yasumasa*; Kitao, Akio*; Shibata, Kaoru; Tokuhisa, Atsushi*; Tsukushi, Itaru*; Go, Nobuhiro
Physica B; Condensed Matter, 385-386(2), p.871 - 873, 2006/11
Times Cited Count:13 Percentile:52.05(Physics, Condensed Matter)The boson peak of a protein was examined in relation to hydration using staphylococcal nuclease. Although the boson peak is commonly observed in synthetic polymers, glassy materials and amorphous materials, the origin of the boson peak is not fully understood. The motions that contribute to the peak are harmonic vibrations. Upon hydration the peak frequency shifts to a higher frequency and the effective force constant of the vibration increases at low temperatures, suggesting that the protein energy surface is modified. Hydration of the protein leads to a more rugged surface and the vibrational motions are trapped within the local minimum at cryogenic temperatures. The origin of the protein boson peak may be related to this rugged energy surface.
Kitao, Akio*; Yonekura, Koji*; Yonekura, Saori*; Samatey, F.*; Imada, Katsumi*; Namba, Keiichi*; Go, Nobuhiro
Proceedings of the National Academy of Sciences of the United States of America, 103(13), p.4894 - 4899, 2006/03
Times Cited Count:45 Percentile:63.3(Multidisciplinary Sciences)no abstracts in English
Ishida, Hisashi; Higuchi, Mariko; Yonetani, Yoshiteru*; Kano, Takuma; Jochi, Yasumasa*; Kitao, Akio*; Go, Nobuhiro
Annual Report of the Earth Simulator Center April 2004 - March 2005, p.241 - 246, 2005/12
no abstracts in English
Jochi, Yasumasa*; Kitao, Akio*; Go, Nobuhiro
Journal of the American Chemical Society, 127(24), p.8705 - 8709, 2005/06
Times Cited Count:27 Percentile:61.19(Chemistry, Multidisciplinary)no abstracts in English
Ishida, Hisashi; Jochi, Yasumasa*; Higuchi, Mariko; Kano, Takuma; Kitao, Akio*; Go, Nobuhiro
Annual Report of the Earth Simulator Center April 2003 - March 2004, p.175 - 179, 2004/07
no abstracts in English
Kitao, Akio
Hamon, 12(2), p.80 - 83, 2002/04
no abstracts in English
Hikuchi, Mariko; Ishida, Hisashi; Kitao, Akio*; Yamagata, Yuriko*; Go, Nobuhiro
no journal, ,
no abstracts in English
Nakagawa, Hiroshi; Jochi, Yasumasa*; Kitao, Akio*; Shibata, Kaoru; Tokuhisa, Atsushi*; Go, Nobuhiro; Kataoka, Mikio
no journal, ,
Protein dynamics in a solvated sample are strongly coupled to their environment. The dynamical transition and boson peak of a protein were examined in relation to hydration using staphylococcal nuclease. A dynamical transition of protein around 230K is observed only for the hydrated protein. It is demonstrated that the functions of some proteins are suppressed with the loss of anharmonic dynamics as the proteins are cooled down below the dynamical transition temperature. Hydration level dependence of the dynamical transition was examined. The dynamical transition was observed at higher hydration level, 0.26gwater/gprotein. The previous work reported that about 0.2 gwater/gprotein hydration is necessary for the protein function. This suggests that dynamical transition is important for protein function. On the other hand, below the 150K, even low hydration affects the harmonic vibration of protein. At low temperature the protein boson peak was observed. Although the boson peak is commonly observed in synthetic polymers, glassy materials and amorphous materials, the origin of the boson peak has not been fully understood. The motions that contribute to the peak are harmonic vibrations. Upon hydration the peak frequency shifts to a higher frequency and the effective force constant of the vibration increases at low temperatures, suggesting that the protein energy surface is modified. Hydration of the protein leads to a more rugged potential surface and the vibrational motions are trapped within a local minimum at cryogenic temperatures. The origin of the protein boson peak is related to this rugged energy surface and the distribution of low-energy vibrations.
Nakagawa, Hiroshi; Jochi, Yasumasa*; Kitao, Akio*; Shibata, Kaoru; Go, Nobuhiro; Kataoka, Mikio
no journal, ,
no abstracts in English
Nakagawa, Hiroshi; Jochi, Yasumasa*; Kitao, Akio*; Shibata, Kaoru; Go, Nobuhiro; Kataoka, Mikio
no journal, ,
no abstracts in English
Nakagawa, Hiroshi; Jochi, Yasumasa*; Kitao, Akio*; Shibata, Kaoru; Go, Nobuhiro; Kataoka, Mikio
no journal, ,
Pico-second dynamics of protein and its hydration water were examined by incoherent neutron inelastic scattering with protein sample at various hydration levels. Hydration affects the protein low frequency modes significantly and brings the shift of the boson peak toward higher energy. At cryogenic temperature, the force constant of the harmonic potential for the protein low frequency mode increases in proportion to the hydration level, which is correlated with the boson peak shift. Theoretical study indicated that the hydration water molecules hydrogen-bonded with a protein make the potential surface of protein low frequency collective mode more rugged. The observed boson peak shift is consistent with the theoretical conclusion. Hydration-dependent protein dynamical transition appears around 240K above the hydration level of h=0.20. At every hydration level examined, the mean square displacement of the hydration water is almost identical to that of the protein up to the dynamical transition temperature. While hydration water shows similar dynamical transition to the protein dynamical transition above the threshold hydration level, hydration water never shows the transition below the threshold hydration. The anomalous dynamical behavior of hydration water should be coupled with the protein collective mode via hydrogen-bond network. Such a dynamical coupling drives the hydration dependent protein dynamical transition.