Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kitamura, Akira; Shibata, Masahiro; Kitao, Hideo*
Materials Research Society Symposium Proceedings, Vol.807, p.609 - 614, 2004/00
None
Kitamura, Akira; Shibata, Masahiro; Kitao, Hideo*
JNC TN8400 2003-004, 76 Pages, 2003/03
Chemical behavior of selenium(Se), which was one of the important elements for performance assessment of geological disposal of high-level radioactive waste, was investigated under reducing and iron-containing conditions. A washing method for an iron diselenide(FeSe (cr)) reagent with acidic and basic solutions (0.1 and 1 M HCl and 1 M NaOH) was carried out for the purification of FeSe
(cr)) reagent with acidic and basic solutions (0.1 and 1 M HCl and 1 M NaOH) was carried out for the purification of FeSe reagent, which was considered to be a solubility limiting solid for Se under the geological disposal conditions. Furthermore, solubility of FeSe
reagent, which was considered to be a solubility limiting solid for Se under the geological disposal conditions. Furthermore, solubility of FeSe (cr) was measured in alkaline solution (pH: 11 - 13) under reducing conditions (E
(cr) was measured in alkaline solution (pH: 11 - 13) under reducing conditions (E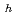 vs SHE: -0.4 - O V), and thermodynamic data on equilibrium reactions between Se in solution and Se precipitate were obtained. The dependencies of solubility values on pH and redox potential (Eh: vs. standard hydrogen electrode) were best interpreted that the solubility limiting solid was not FeSe
vs SHE: -0.4 - O V), and thermodynamic data on equilibrium reactions between Se in solution and Se precipitate were obtained. The dependencies of solubility values on pH and redox potential (Eh: vs. standard hydrogen electrode) were best interpreted that the solubility limiting solid was not FeSe (cr) but Se(cr) and the aqueous species was SeO
(cr) but Se(cr) and the aqueous species was SeO
 in the present experimental conditions. The equilibrium constant between Se(cr) and SeO
in the present experimental conditions. The equilibrium constant between Se(cr) and SeO
 at zero ionic strength was determined and compared with literature values. The chemical behavior of Se under geological disposal conditions was discussed.
at zero ionic strength was determined and compared with literature values. The chemical behavior of Se under geological disposal conditions was discussed.
Kitao, Hideo*; Kogawa, Noritaka*
JNC TJ6420 2003-010, 156 Pages, 2003/02
Yanagisawa, Ichiro*; Katsurai, Kiyomichi*; Izumi, Jun*; Saigusa, Moriyuki*; Kitao, Hideo*; Tsuzuki, Yasuo*; Neyama, Atsushi*; Kato, Hiroyasu*; Nakazawa, Toshiyuki*; Okada, Kenichi*
JNC TJ8400 2000-037, 61 Pages, 2000/02
no abstracts in English
*; Katsurai, Kiyomichi*; Saigusa, Moriyuki*; *; Kitao, Hideo*; Tsuzuki, Yasuo*
JNC TJ8400 2000-012, 333 Pages, 2000/02
In this study, technical review, laboratory experiment and modeling calculations were implemented in order to reliability of geochemical assessment technique for the second progress report. The summary of this study is as follows: (1)We have implemented the technical review of the second progress backup report geochemical modeling and some data. We have extracted a subject about groundwater evolution modeling and thermodynamic data. (2)We have implemented multivariable analysis based on database of deep groundwater sampled in geoscientofic investigation in other countries. We have considered validity of peculiar model groundwater of SRLP, FRLP and MRNP. (3)We have researched on redox reaction of a coastal sedimentary layer, and was cleared geochemical mechanism of dedox reaction. (4)We have studied thermodynamic of Se in laboratory. We have confirmed condition, which SRB was not in experimental system, initial solid transformed to solid phase (FeSe). And we have clarified chemical species of Se in the liquid phase. (5)We have researched on degradation of the engineered barrier material. We have prepared natural analogue data set that related to the iron and cooper. And we have acquired basic property of buffer material (Kunipia-F) in laboratory. (6)We have studied on dissolution kinetic of UO in order to extract geochemistry technical factor for site selection. The dissolution rates in oxidized groundwater may be much slower than previously believed by most scientists. Therefore, oxidized environments, as well as reduced environments, may be considered as possible sites for underground repository sites.
in order to extract geochemistry technical factor for site selection. The dissolution rates in oxidized groundwater may be much slower than previously believed by most scientists. Therefore, oxidized environments, as well as reduced environments, may be considered as possible sites for underground repository sites.
*; Katsurai, Kiyomichi*; Saigusa, Moriyuki*; *; Kitao, Hideo*; Tsuzuki, Yasuo*
JNC TJ8400 2000-011, 65 Pages, 2000/02
no abstracts in English
Tachikawa, Hirokazu*; Kitao, Hideo*; Katsurai, Kiyomichi*; Yanagisawa, Ichiro*; Shibata, Masahiro; Suyama, Tadahiro*; Yui, Mikazu
JNC TN8400 99-068, 108 Pages, 1999/11
In an evaluation of high level waste (HLW) repository performance, Se-79 is one of important elements to be analyzed. Selenium solubility and solubility limiting solid phase is not clear. Then, we performed solubility measurement tests from over saturation direction under reducing conditions considering the repository conditions in deep underground. In some cases, bentonite (Kunigel V1) or pyrite coexisted in the experimental system to simulate the repository conditions. Se bearing solids were determined by XRD analysis, and solubility limiting solid phase was discussed. FeSe (Ferroselite) and Se (hexagonal) were identified in the simple condition test, in which Fe(II) solution and Se solution were mixed. SeS was also identified when S(-II) solution was added. The Se concentrations in aqueous phase were approximately 10
(Ferroselite) and Se (hexagonal) were identified in the simple condition test, in which Fe(II) solution and Se solution were mixed. SeS was also identified when S(-II) solution was added. The Se concentrations in aqueous phase were approximately 10 mol/l at neutral pH and approximately 10
mol/l at neutral pH and approximately 10 mol/l at pH9 in the bentonite coexisting tests and pyrite coexisting tests. The solid phases identified in the pyrite coexisting tests were mainly Se(hexagonal) and FeSe
mol/l at pH9 in the bentonite coexisting tests and pyrite coexisting tests. The solid phases identified in the pyrite coexisting tests were mainly Se(hexagonal) and FeSe (Ferroselite) in one of the samples. Further, the possibility of Fe(S,Se) solid solution formation was presumed on the pyrite surface dipping in the test solutions. In addition, we performed another selenium solubility measurement to accelerate the transformation of Se bearing solid phase at elevated temperature (80
(Ferroselite) in one of the samples. Further, the possibility of Fe(S,Se) solid solution formation was presumed on the pyrite surface dipping in the test solutions. In addition, we performed another selenium solubility measurement to accelerate the transformation of Se bearing solid phase at elevated temperature (80 C). The concentration of Se decreases with time and reached to the detection limit of ICP-MS (4
C). The concentration of Se decreases with time and reached to the detection limit of ICP-MS (4 10
10 mol/1) in 3 months. At first, Se(hexagonal) is dominant in the precipitation, however this solid phase was gradually transformed to Fe-Se solids (FeSe, FeSe
mol/1) in 3 months. At first, Se(hexagonal) is dominant in the precipitation, however this solid phase was gradually transformed to Fe-Se solids (FeSe, FeSe ) with time. Therefore it is strongly suggested that FeSe
) with time. Therefore it is strongly suggested that FeSe which is the thermodynamically most stable phase will be a solubility limiting solid phase under repository conditions in long term. As the experimental system was confirmed as sulfate reducing bacteria free, it should be noted that whole observed reactions ...
which is the thermodynamically most stable phase will be a solubility limiting solid phase under repository conditions in long term. As the experimental system was confirmed as sulfate reducing bacteria free, it should be noted that whole observed reactions ...
Yanagisawa, Ichiro*; Naito, Taisei*; Kitao, Hideo*; Mukai, Satoru*; Doi, Hideo*
JNC TJ8400 99-020, 25 Pages, 1999/02
In this study, apparent diffusion coefficients and distribution coefficient of some nuclides were measured for bentonite materials as engineered barrier. The summary of this study is as follows: (1)Apparent diffusion coefficients of nuclides in compacted bentonite were measured. The data so far were summarized as database of apparent diffusion coefficients and distribution coefficients. (2)In diffusion tests for mixture of Ca-type altered bentonite and analcime, apparent diffusion coefficient of Sr was a little smaller than that for Ca-type altered bentonite. (3)In diffusion tests for Ca-type altered bentonite, apparent diffusion coefficients and distribution coefficients of nuclides showed no significant difference between bentonite-water system and bentonite-cementitious solution systems. (4)In Na-type bentonite-solution with nitrate system, apparent diffusion coefficients of Cs and Sr were smaller than that for other bentonite-solution systems. (5)Apparent diffusion coefficients of tritium showed no significant difference between Ca-type altered bentonite and Na-type bentonite in any solution systems.
Kataoka, Shinichi*; Kitao, Hideo*; Tachikawa, Hirokazu*; Shimada, Takashi*; Maeda, Kazuto*; Nemoto, Kazuaki*; Yanagisawa, Ichiro*
PNC TJ1216 98-002, 676 Pages, 1998/02
None
Iriya, Keishiro*; Kubo, Hiroshi*; *; Mukai, Satoru*; Kitao, Hideo*; Ishihara, Yoshinao*; Neyama, Atsushi*
PNC TJ1449 96-002, 71 Pages, 1996/03
None
Iriya, Keishiro*; Kubo, Hiroshi*; *; Mukai, Satoru*; Kitao, Hideo*; Ishihara, Yoshinao*; Neyama, Atsushi*
PNC TJ1449 96-001, 379 Pages, 1996/03
None
Mukai, Satoru*; Kitao, Hideo*; Tachikawa, Hirokazu*; Fusaeda, Shigeki*; Yanagisawa, Ichiro*; Doi, Motoo*; *
PNC TJ1216 96-003, 106 Pages, 1996/03
None