Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Emori, Tatsuya; Kitatsuji, Yoshihiro; Ban, Yasutoshi
JAEA-Technology 2024-025, 20 Pages, 2025/03
Radioisotope Thermoelectric Generators (RTGs) using the decay heat of Pu-238 has been applied for outer planet missions far from Jupiter, where solar power is limited. However, no facilities are available to produce Pu-238 for space probes in Japan. Moreover, the use of nuclear materials for the space exploration is difficult in term of the regulation. Thus, we focused on Am-241 whose half-life is around 432 years as an alternative heat source for RTGs. This report describes the procedure of separating Am-241 decayed from Pu-241 in aged plutonium oxide. Two experiments were performed: one using solid-liquid extraction and the other combining liquid-liquid extraction and solid-liquid extraction. Packed columns were used in the experiments, with their number reduced by less than one-fifth in the latter experiment compared to the former. Furthermore, the time required for separation in the latter experiment was less than half that of the former. We performed the separation experiments six times, collecting a total of approximately 0.43 g of Am-241 as an oxalate salt.
Seito, Hajime*; Yokozuka, Eri*; Oka, Toshitaka; Kitatsuji, Yoshihiro; Nagasawa, Naotsugu*
Radiation Protection Dosimetry, 200(16-18), p.1656 - 1659, 2024/11
Times Cited Count:0 Percentile:0.00(Environmental Sciences)We have examined dosimetric characteristics of bio-inspired carbonated hydroxyapatite (CO HAp), which is a main component of calcified tissues like tooth enamel. CO
HAp), which is a main component of calcified tissues like tooth enamel. CO HAp powder samples were exposed to gamma-ray with radiation doses ranging from 1 Gy to 1000 Gy at room temperature, and ESR spectra were measured immediately after irradiation and subsequently measured each 1 day during 90 days. The post-irradiation fading resulted in significant 20% decay of the signal amplitude, which stabilised within 7 days after irradiation, and the intensity approached a constant. The sample has good linear dose response in the experiment range of 10 Gy - 1000 Gy. Our results indicate that the CO
HAp powder samples were exposed to gamma-ray with radiation doses ranging from 1 Gy to 1000 Gy at room temperature, and ESR spectra were measured immediately after irradiation and subsequently measured each 1 day during 90 days. The post-irradiation fading resulted in significant 20% decay of the signal amplitude, which stabilised within 7 days after irradiation, and the intensity approached a constant. The sample has good linear dose response in the experiment range of 10 Gy - 1000 Gy. Our results indicate that the CO HAp materials is suitable for chemical dosimetry.
HAp materials is suitable for chemical dosimetry.
Kitamura, Yoshimasa; Oka, Toshitaka; Seito, Hajime*; Yokozuka, Eri*; Nagasawa, Naotsugu*; Kitatsuji, Yoshihiro
Radiation Protection Dosimetry, 200(16-18), p.1660 - 1665, 2024/11
Times Cited Count:0 Percentile:0.00(Environmental Sciences)In this work, we evaluated the applicability of hydroxyapatite, which is a main component of tooth enamel, as individual dosimeters that can detect from less than 1 Gy to several tens Gy. Commercially available hydroxyapatite was irradiated by  Co gamma-ray up to 75 Gy and ESR spectrum of the irradiated sample was observed. The relationship between the intensity of produced carbonated radical and the absorbed dose shows a good linearity (
Co gamma-ray up to 75 Gy and ESR spectrum of the irradiated sample was observed. The relationship between the intensity of produced carbonated radical and the absorbed dose shows a good linearity (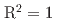 ) from 0 to 75 Gy. The detection limit of this samples was estimated to be 99.7 mGy, and the radical intensity do not change for eight month from the irradiation. These results suggest that this sample can be used as a candidate of the individual dosimeter.
) from 0 to 75 Gy. The detection limit of this samples was estimated to be 99.7 mGy, and the radical intensity do not change for eight month from the irradiation. These results suggest that this sample can be used as a candidate of the individual dosimeter.
Yomogida, Takumi; Ouchi, Kazuki; Morii, Shiori; Oka, Toshitaka; Kitatsuji, Yoshihiro; Koma, Yoshikazu; Konno, Katsuhiro*
Scientific Reports (Internet), 14, p.14945_1 - 14945_11, 2024/06
Times Cited Count:1 Percentile:31.14(Multidisciplinary Sciences)Particles containing alpha ( ) nuclides were identified from sediment in stagnant water in the Unit 3 reactor building of the Fukushima Daiichi Nuclear Power Station (FDiNPS). We analyzed different concentrations of alpha nuclides samples collected at two sampling sites, torus room and Main steam isolation valve (MSIV) room. Most of the
) nuclides were identified from sediment in stagnant water in the Unit 3 reactor building of the Fukushima Daiichi Nuclear Power Station (FDiNPS). We analyzed different concentrations of alpha nuclides samples collected at two sampling sites, torus room and Main steam isolation valve (MSIV) room. Most of the  -nuclides in the stagnant water samples of the torus room and the MSIV room were present in particle fractions larger than 10
-nuclides in the stagnant water samples of the torus room and the MSIV room were present in particle fractions larger than 10  m. We detected uranium-bearing particles in
m. We detected uranium-bearing particles in  m-size by scanning electron microscopy-energy dispersive X-Ray (SEM-EDX) observation. Other short lived
m-size by scanning electron microscopy-energy dispersive X-Ray (SEM-EDX) observation. Other short lived  -nuclides were detected by alpha track detection. The
-nuclides were detected by alpha track detection. The  -nuclide-containing particles with several tens to several hundred
-nuclide-containing particles with several tens to several hundred  m in size were mainly comprised iron (Fe) by SEM-EDX analysis. This study clarifies that the morphologies of U and other
m in size were mainly comprised iron (Fe) by SEM-EDX analysis. This study clarifies that the morphologies of U and other  -nuclides in the sediment of stagnant water in the FDiNPS's Unit 3 reactor building.
-nuclides in the sediment of stagnant water in the FDiNPS's Unit 3 reactor building.
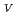 O
O
 /U
/U O
O
 redox equilibrium in [emim]Tf
redox equilibrium in [emim]Tf N-based liquid electrolytes towards uranium redox-flow battery
N-based liquid electrolytes towards uranium redox-flow batteryTakao, Koichiro*; Ouchi, Kazuki; Komatsu, Atsushi; Kitatsuji, Yoshihiro; Watanabe, Masayuki
European Journal of Inorganic Chemistry, 27(14), p.e202300787_1 - e202300787_7, 2024/05
Times Cited Count:1 Percentile:0.00(Chemistry, Inorganic & Nuclear)Electrochemical behavior of U O
O
 /U
/U O
O
 in 1-ethyl-3-methylimidazolium bis(trifluoromethyl)sulfonylamide ([emim]Tf
in 1-ethyl-3-methylimidazolium bis(trifluoromethyl)sulfonylamide ([emim]Tf N) ionic liquid was studied to clarify what are required to attain its redox reversibility for utilizing depleted U as an electrode active material in a redox flow battery. As a result, reversibility of the U
N) ionic liquid was studied to clarify what are required to attain its redox reversibility for utilizing depleted U as an electrode active material in a redox flow battery. As a result, reversibility of the U O
O
 /U
/U O
O
 redox reaction was successfully achieved in use of a glassy carbon working electrode under presence of Cl
redox reaction was successfully achieved in use of a glassy carbon working electrode under presence of Cl in [emim]Tf
in [emim]Tf N. To improve diffusivity of solutes, [emim]Tf
N. To improve diffusivity of solutes, [emim]Tf N diluted with an auxiliary molecular solvent, N,N-dimethylformamide (DMF). We have succeeded in demonstrating a reversible redox reaction of [U
N diluted with an auxiliary molecular solvent, N,N-dimethylformamide (DMF). We have succeeded in demonstrating a reversible redox reaction of [U O
O Cl
Cl ]
] + e
+ e = [U
= [U O
O Cl
Cl ]
] in the 50:50 v/v [emim]Tf
in the 50:50 v/v [emim]Tf N-DMF liquid electrolyte containing Cl
N-DMF liquid electrolyte containing Cl .
.
 Ac and its uncertainty through the
Ac and its uncertainty through the  Ra(n,2n) reaction in the experimental fast reactor Joyo
Ra(n,2n) reaction in the experimental fast reactor JoyoSasaki, Yuto*; Sano, Aaru; Sasaki, Shinji; Iwamoto, Nobuyuki; Ouchi, Kazuki; Kitatsuji, Yoshihiro; Takaki, Naoyuki*; Maeda, Shigetaka
Journal of Nuclear Science and Technology, 61(4), p.509 - 520, 2024/04
Times Cited Count:5 Percentile:78.63(Nuclear Science & Technology) Ac is attracting attention as an alpha-emitting medical radioisotope. Since its demand is expected to increase, domestic production of
Ac is attracting attention as an alpha-emitting medical radioisotope. Since its demand is expected to increase, domestic production of  Ac is required from the viewpoint of Japan's medical research and economic security. To establish the technical bases for the
Ac is required from the viewpoint of Japan's medical research and economic security. To establish the technical bases for the  Ac production, JAEA has evaluated the radioactivity that can be produced in the experimental fast reactor Joyo and designed the concept that upgrades the existing facilities for transporting the irradiated target from Joyo to a neighboring PIE facility rapidly. Efficient
Ac production, JAEA has evaluated the radioactivity that can be produced in the experimental fast reactor Joyo and designed the concept that upgrades the existing facilities for transporting the irradiated target from Joyo to a neighboring PIE facility rapidly. Efficient  Ac Separation from
Ac Separation from  Ra irradiated in a fast reactor was studied. Ba and La were used as alternatives to Ra and Ac, respectively. By using DGA resin as an adsorbent, it can be expected that Ra and impurities generated by irradiation will be removed and Ac will be isolated. This study has revealed that Joyo can sufficiently produce
Ra irradiated in a fast reactor was studied. Ba and La were used as alternatives to Ra and Ac, respectively. By using DGA resin as an adsorbent, it can be expected that Ra and impurities generated by irradiation will be removed and Ac will be isolated. This study has revealed that Joyo can sufficiently produce  Ac as a raw material for pharmaceuticals.
Ac as a raw material for pharmaceuticals.
Yomogida, Takumi; Hashimoto, Tadashi; Okumura, Takuma*; Yamada, Shinya*; Tatsuno, Hideyuki*; Noda, Hirofumi*; Hayakawa, Ryota*; Okada, Shinji*; Takatori, Sayuri*; Isobe, Tadaaki*; et al.
Analyst, 149(10), p.2932 - 2941, 2024/03
Times Cited Count:1 Percentile:34.56(Chemistry, Analytical)In this study, we successfully applied a transition-edge sensor (TES) spectrometer as a detector for microbeam X-ray measurements from a synchrotron X-ray light source to determine uranium (U) distribution at the micro-scale and its chemical species in biotite obtained from the U mine. It is difficult to separate the fluorescent X-ray of the U L
 line at 13.615 keV from that of the Rb K
line at 13.615 keV from that of the Rb K line at 13.395 keV in the X-ray fluorescence spectrum with an energy resolution of approximately 220 eV of the conventional silicon drift detector (SDD). Meanwhile, the fluorescent X-rays of U L
line at 13.395 keV in the X-ray fluorescence spectrum with an energy resolution of approximately 220 eV of the conventional silicon drift detector (SDD). Meanwhile, the fluorescent X-rays of U L
 and Rb K
and Rb K were fully separated by TES with 50 eV energy resolution at the energy of around 13 keV. The successful peak separation by TES led to an accurate mapping analysis of trace U in micro-X-ray fluorescence measurements and a decrease in the signal-to-background ratio in micro-X-ray absorption near edge structure spectroscopy.
were fully separated by TES with 50 eV energy resolution at the energy of around 13 keV. The successful peak separation by TES led to an accurate mapping analysis of trace U in micro-X-ray fluorescence measurements and a decrease in the signal-to-background ratio in micro-X-ray absorption near edge structure spectroscopy.
Morii, Shiori; Yomogida, Takumi; Asai, Shiho*; Ouchi, Kazuki; Oka, Toshitaka; Kitatsuji, Yoshihiro
KEK Proceedings 2023-2, p.132 - 137, 2023/11
New analytical method of a combination of laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) and isotope dilution mass spectrometry (IDMS) for quantification of Zr isotopes in a solid sample was investigated. Solid Zr-isotope reference was added to a simulated radioactive waste sample as a spike, then Zr isotope ratio was measured by LA-ICP-MS. As a result, we successfully quantify Zr isotopes in the simulated radioactive waste sample by new IDMS. There is a possibility that this new method can be applied for quantification of Zr-93 in difficult to dissolve radioactive wastes.
Morii, Shiori; Yomogida, Takumi; Asai, Shiho*; Ouchi, Kazuki; Oka, Toshitaka; Kitatsuji, Yoshihiro
Bunseki Kagaku, 72(10.11), p.441 - 448, 2023/10
Rapid analytical method for the determination of Zr-93 in radioactive wastes has been developed. Laser ablation (LA)-ICP-MS was applied to the analysis of Zr isotopes in simulated high-level radioactive waste (HLW). Sample preparation time was dramatically reduced by using a DGA resin as the adsorbent for Zr. Direct quantification of Zr isotopes in this resin sample was carried out by LA-ICP-MS. Laser settings were optimized to obtain a reliable isotope ratio of the sample by LA-ICP-MS. Quantification of Zr isotopes in the simulated HLW solution by isotope dilution mass spectrometry (IDMS) was examined. The amount of Zr-90 in the sample obtained by IDMS corresponded to a value calculated from the given concentration of Zr in the sample within uncertainty. Thus, this method can be applied for the quantification of Zr-93 in radioactive wastes.
 dissolution in bicarbonate solution with H
dissolution in bicarbonate solution with H O
O ; The Effect of temperature
; The Effect of temperatureMcGrady, J.; Kumagai, Yuta; Kitatsuji, Yoshihiro; Kirishima, Akira*; Akiyama, Daisuke*; Watanabe, Masayuki
RSC Advances (Internet), 13(40), p.28021 - 28029, 2023/09
Times Cited Count:2 Percentile:21.35(Chemistry, Multidisciplinary)Upon nuclear waste canister failure and contact of spent nuclear fuel with groundwater, the UO matrix of spent fuel will interact with oxidants in the groundwater generated by water radiolysis. Bicarbonate (HCO
matrix of spent fuel will interact with oxidants in the groundwater generated by water radiolysis. Bicarbonate (HCO
 ) is often found in groundwater, and the H
) is often found in groundwater, and the H O
O induced oxidative dissolution of UO
induced oxidative dissolution of UO in bicarbonate solution has previously been studied under various conditions. Temperatures in the repository at the time of canister failure will differ depending on the location, yet the effect of temperature on oxidative dissolution is unknown. To investigate, the decomposition rate of H
in bicarbonate solution has previously been studied under various conditions. Temperatures in the repository at the time of canister failure will differ depending on the location, yet the effect of temperature on oxidative dissolution is unknown. To investigate, the decomposition rate of H O
O at the UO
at the UO surface and dissolution of U
surface and dissolution of U in bicarbonate solution (0.1, 1, 10 and 50 mM) was analysed at various temperatures (10, 25, 45 and 60
in bicarbonate solution (0.1, 1, 10 and 50 mM) was analysed at various temperatures (10, 25, 45 and 60  C). At [HCO
C). At [HCO
 ]
] 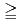 1 mM, the apparent equilibrium concentration of U
1 mM, the apparent equilibrium concentration of U decreased with increasing temperature. This was attributed to the formation of U
decreased with increasing temperature. This was attributed to the formation of U -bicarbonate species at the surface and a change in the mechanism of H
-bicarbonate species at the surface and a change in the mechanism of H O
O decomposition from oxidative to catalytic. At 0.1 mM, no obvious correlation between temperature and U dissolution was observed, and thermodynamic calculations indicated this was due to a change in the surface species. A pathway to explain the observed dissolution behaviour of UO
decomposition from oxidative to catalytic. At 0.1 mM, no obvious correlation between temperature and U dissolution was observed, and thermodynamic calculations indicated this was due to a change in the surface species. A pathway to explain the observed dissolution behaviour of UO in bicarbonate solution as a function of temperature was proposed.
in bicarbonate solution as a function of temperature was proposed.
Horiguchi, Naoki; Yoshida, Hiroyuki; Kitatsuji, Yoshihiro; Hasegawa, Makoto*; Kishimoto, Tadafumi*
Proceedings of 30th International Conference on Nuclear Engineering (ICONE30) (Internet), 7 Pages, 2023/05
From the viewpoint of energy security in Japan and reduction of the environmental load, continuous operation of light water reactors is essential. Since a pH adjuster with enriched Li-7 ions is required for water quality control on PWR, the development of Li-7 enrichment technology is one of the key issues. The multi-channel counter-current electrophoresis (MCCCE) method has been developed as the technology with a low environmental load. To put this method into practical use, it is necessary to understand Li-7 ion behavior in the channel flow and optimize the experimental condition to separate Li-7 and its isotope. In this paper, to understand Li-7 ion behavior in a single channel of the experimental apparatus, a numerical simulation method based on a computational fluid dynamics (CFD) code with a particle tracking method, TPFIT-LPT, was developed. In the method, the motion of multiple ions under the electric field was simulated as a particle with an added velocity by the electric field. The difference in the isotopes was represented by changing of the magnitude of the added velocity. We also considered that although it is impossible to measure the behavior of each ion, it is important to measure the flow velocity of the bulk fluid for the validation of the numerical simulation. We developed a lab-scale experimental apparatus in which the single channel of the actual apparatus was simplified to measure the flow velocity by Particle Image Velocimetry (PIV). We set a pulsation flow condition on the lab-scale experiment, which is one of difficult conditions for the numerical simulation, and measured the velocity. As the result, we confirmed that the pulsation flow was reproduced. We set the measured data as the inlet boundary condition of the numerical simulation and conducted it. As the numerical result, we confirmed the ions affected by the electric field moved upstream with pulsation. We also confirmed the effect of the electric field on the motion of the isotope.
Ouchi, Kazuki; Matsumura, Daiju; Tsuji, Takuya; Kobayashi, Toru; Otobe, Haruyoshi; Kitatsuji, Yoshihiro
RSC Advances (Internet), 13(24), p.16321 - 16326, 2023/05
Times Cited Count:1 Percentile:10.11(Chemistry, Multidisciplinary)We clarified the chemical state transformation of deposits following the reduction of uranyl ion (U O
O
 ) from the results of electrochemical quartz crystal microbalance, impedance spectra and X-ray absorption fine structure measurements. We propose the following deposition mechanism: (1) U
) from the results of electrochemical quartz crystal microbalance, impedance spectra and X-ray absorption fine structure measurements. We propose the following deposition mechanism: (1) U is formed by the disproportionation of U
is formed by the disproportionation of U . (2) U
. (2) U forms U
forms U hydroxide deposits, and (3) finally, the hydroxide deposits transform into U
hydroxide deposits, and (3) finally, the hydroxide deposits transform into U oxide, generally having a larger electrical resistance than the former.
oxide, generally having a larger electrical resistance than the former.
 -edge to assess the U(V) electronic structure in FeUO
-edge to assess the U(V) electronic structure in FeUO
Yomogida, Takumi; Akiyama, Daisuke*; Ouchi, Kazuki; Kumagai, Yuta; Higashi, Kotaro*; Kitatsuji, Yoshihiro; Kirishima, Akira*; Kawamura, Naomi*; Takahashi, Yoshio*
Inorganic Chemistry, 61(50), p.20206 - 20210, 2022/12
Times Cited Count:7 Percentile:58.38(Chemistry, Inorganic & Nuclear)FeUO was studied to clarify the electronic structure of U(V) in a metal monouranate compound. We obtained the peak splitting of HERFD-XANES spectra utilizing high-energy-resolution fluorescence detection-X-ray absorption near edge structure (HERFD-XANES) spectroscopy at the U L
was studied to clarify the electronic structure of U(V) in a metal monouranate compound. We obtained the peak splitting of HERFD-XANES spectra utilizing high-energy-resolution fluorescence detection-X-ray absorption near edge structure (HERFD-XANES) spectroscopy at the U L -edge, which is a novel technique in the U(V) compounds. Theoretical calculations revealed that the peak splitting was caused by splitting the 6d orbital of U(V). Such distinctive electronic states are of major interest to researchers and engineers working in various fields, from fundamental physics to the nuclear industry and environmental sciences for actinide elements.
-edge, which is a novel technique in the U(V) compounds. Theoretical calculations revealed that the peak splitting was caused by splitting the 6d orbital of U(V). Such distinctive electronic states are of major interest to researchers and engineers working in various fields, from fundamental physics to the nuclear industry and environmental sciences for actinide elements.
Yomogida, Takumi; Kitatsuji, Yoshihiro; Miyamoto, Yutaka
KEK Proceedings 2022-2, p.148 - 153, 2022/11
The Research Group for Safeguards Analytical Chemistry is currently developing a method to analyze the chemical state of uranium particles in environmental samples collected at nuclear facilities using micro-Raman spectroscopy. The chemical state of uranium particles in environmental samples can be partially oxidized by long-term exposure to air. It is necessary to develop a method to analyze the chemical state of the entire particle. In this study, uranium dioxide stored under atmospheric conditions was analyzed by micro-Raman mapping. The Raman spectra showed that uranium peroxide was locally present in the UO particle. The Raman peaks originating from the structure of UO
particle. The Raman peaks originating from the structure of UO around 570 cm
around 570 cm and 1150 cm
and 1150 cm could not be observed in the point analysis of the particle center. On the other hand, in mapping analysis, Raman peaks originating from the structure of UO
could not be observed in the point analysis of the particle center. On the other hand, in mapping analysis, Raman peaks originating from the structure of UO can be observed from the same particle, demonstrating that Raman mapping analysis is an effective method for analyzing the chemical state of the entire particle.
can be observed from the same particle, demonstrating that Raman mapping analysis is an effective method for analyzing the chemical state of the entire particle.
 Ac for Targeted Alpha Therapy (TAT) using the experimental fast reactor Joyo
Ac for Targeted Alpha Therapy (TAT) using the experimental fast reactor JoyoMaeda, Shigetaka; Kitatsuji, Yoshihiro
Enerugi Rebyu, 42(10), p.19 - 22, 2022/09
Ac-225 is attracting attention as an alpha-emitting medical radioisotope. Since its demand is expected to increase, domestic production of Ac-225 is required from the viewpoint of Japan's medical research and economic security. To establish the technical bases for the Ac-225 production, JAEA has evaluated the radioactivity that can be produced in the experimental fast reactor Joyo and designed the concept that upgrades the existing facilities for transporting the irradiated target from Joyo to a neighboring PIE facility rapidly. Efficient Actinium-225 Separation from Ra-226 irradiated in a fast reactor was studied. Ba and La were used as alternatives to Ra and Ac, respectively. By using DGA resin as an adsorbent, it can be expected that Ra and impurities generated by irradiation will be removed and Ac will be isolated. This study has revealed that Joyo can sufficiently produce Ac-225 as a raw material for pharmaceuticals.
Yomogida, Takumi; Ouchi, Kazuki; Oka, Toshitaka; Kitatsuji, Yoshihiro; Koma, Yoshikazu; Konno, Katsuhiro*
Scientific Reports (Internet), 12(1), p.7191_1 - 7191_10, 2022/05
Times Cited Count:9 Percentile:55.41(Multidisciplinary Sciences)Particles containing alpha ( ) nuclides were identified from sediment in stagnant water at the torus room of the Fukushima Dai-ichi Nuclear Power Station (FDiNPS)'s Unit 2 reactor. Several uranium-bearing particles were identified by SEM observation. These particles contained Zr and other elements which constituted fuel cladding and structural materials. The
) nuclides were identified from sediment in stagnant water at the torus room of the Fukushima Dai-ichi Nuclear Power Station (FDiNPS)'s Unit 2 reactor. Several uranium-bearing particles were identified by SEM observation. These particles contained Zr and other elements which constituted fuel cladding and structural materials. The  U/
U/ U isotope ratio in the solid fractions that included U particles was consistent with the nuclear fuel in the Unit 2 reactor, which indicated that the U particles had been derived from nuclear fuel. The particles with alpha-emitters detected by alpha track analysis were several tens to several hundred
U isotope ratio in the solid fractions that included U particles was consistent with the nuclear fuel in the Unit 2 reactor, which indicated that the U particles had been derived from nuclear fuel. The particles with alpha-emitters detected by alpha track analysis were several tens to several hundred  m in size. The EDX spectra showed that these particles mainly comprised iron, which indicated Pu, Am, and Cm were adsorbed on the Fe-baring particles. This study clarifies that the major morphologies of U and other
m in size. The EDX spectra showed that these particles mainly comprised iron, which indicated Pu, Am, and Cm were adsorbed on the Fe-baring particles. This study clarifies that the major morphologies of U and other  -nuclides were differed in the sediment of stagnant water in the torus room of FDiNPS's Unit 2 reactor.
-nuclides were differed in the sediment of stagnant water in the torus room of FDiNPS's Unit 2 reactor.
Sato, Nobuaki*; Kirishima, Akira*; Watanabe, Masayuki; Sasaki, Takayuki*; Uehara, Akihiro*; Takeda, Shino*; Kitatsuji, Yoshihiro; Otobe, Haruyoshi; Kobayashi, Taishi*
The Chemistry of Thorium, Plutonium and MA, 254 Pages, 2022/03
The chemistry of nuclear materials such as Thorium (Part 1) and Plutonium (Part 2) was described in relation from the fundamentals on solid chemistry and solution chemistry to the practicals on the experiment and evaluation method in detail. Minor actinides such as Neptunium, Americium, Curium and Protoactinium, was introduced the basics on the solid and solution chemistry.
Ouchi, Kazuki; Tsukahara, Takehiko*; Brandt, A.*; Muto, Yuki*; Nabatame, Nozomi*; Kitatsuji, Yoshihiro
Analytical Sciences, 37(12), p.1789 - 1794, 2021/12
Times Cited Count:1 Percentile:3.74(Chemistry, Analytical)We attempted to scale down a separation process of uranium (U) using the microchip column loaded with anion exchange resin to develop safety and waste-reducing separation technique. The ideal separation performance of U was obtained by the properly design of a microchannel. The concentration of U in seawater as a real-world sample could be quantified with the prepared microchip column. It indicates that the microchip column is sufficiently practical. Compared to separation of U with a general column, the column size was successfully scaled down to  1/5000.
1/5000.
Yomogida, Takumi; Saeki, Morihisa*; Morii, Shiori; Oba, Hironori*; Kitatsuji, Yoshihiro
Analytical Sciences, 37(12), p.1843 - 1846, 2021/12
Times Cited Count:1 Percentile:3.74(Chemistry, Analytical)In this study, we developed a simple and one-step Pd separation technique based on photoreduction with Xe lamp irradiation for the determination of  Pd in highly radioactive samples. A simulated high-level radioactive liquid wastes (HLLW) solution, which consists of 14 major elements (Rb, Sr, Zr, Mo, Ru, Rh, Pd, Cs, Ba, La, Ce, Pr, Nd, Sm) in a 3 mol L
Pd in highly radioactive samples. A simulated high-level radioactive liquid wastes (HLLW) solution, which consists of 14 major elements (Rb, Sr, Zr, Mo, Ru, Rh, Pd, Cs, Ba, La, Ce, Pr, Nd, Sm) in a 3 mol L HNO
HNO solution, was used to evaluate the separation performance. The Pd precipitate were formed by Xe lamp irradiation and recovered by centrifugation. The results showed that the recovery of Pd from a simulated HLLW solution depend on the irradiation time and concentration of ethanol. By optimizing the conditions at photo irradiation, the Pd recovery from the simulated HLLW solution reached up to 50 %, while 99.5 % of the other 13 elements were separated. The Pd precipitate could be separated from the elements that are the main source of radioactivity (Sr, Cs, and Ba) and the source of spectral interference for the determination of
solution, was used to evaluate the separation performance. The Pd precipitate were formed by Xe lamp irradiation and recovered by centrifugation. The results showed that the recovery of Pd from a simulated HLLW solution depend on the irradiation time and concentration of ethanol. By optimizing the conditions at photo irradiation, the Pd recovery from the simulated HLLW solution reached up to 50 %, while 99.5 % of the other 13 elements were separated. The Pd precipitate could be separated from the elements that are the main source of radioactivity (Sr, Cs, and Ba) and the source of spectral interference for the determination of  Pd (Zr, and Ru). These results indicate that selective separation of Pd is achieved with the proposed method, showing the applicability of the proposed separation technique to HLLW samples.
Pd (Zr, and Ru). These results indicate that selective separation of Pd is achieved with the proposed method, showing the applicability of the proposed separation technique to HLLW samples.
Inagawa, Jun; Kitatsuji, Yoshihiro; Otobe, Haruyoshi; Nakada, Masami; Takano, Masahide; Akie, Hiroshi; Shimizu, Osamu; Komuro, Michiyasu; Oura, Hirofumi*; Nagai, Isao*; et al.
JAEA-Technology 2021-001, 144 Pages, 2021/08
Plutonium Research Building No.1 (Pu1) was qualified as a facility to decommission, and preparatory operations for decommission were worked by the research groups users and the facility managers of Pu1. The operation of transportation of whole nuclear materials in Pu1 to Back-end Cycle Key Element Research Facility (BECKY) completed at Dec. 2020. In the operation included evaluation of criticality safety for changing permission of the license for use nuclear fuel materials in BECKY, cask of the transportation, the registration request of the cask at the institute, the test transportation, formulation of plan for whole nuclear materials transportation, and the main transportation. This report circumstantially shows all of those process to help prospective decommission.