Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O
O , FeUO
, FeUO , and UO
, and UO -Zr-stainless steel system samples generated in an oxidative atmosphere in the presence of malonic acid
-Zr-stainless steel system samples generated in an oxidative atmosphere in the presence of malonic acidTonna, Ryutaro*; Sasaki, Takayuki*; Okamoto, Yoshihiro; Kobayashi, Taishi*
Journal of Nuclear Materials, 605, p.155561_1 - 155561_9, 2025/02
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Shimomura, Koichiro*; Koda, Akihiro*; Pant, A. D.*; Sunagawa, Hikaru*; Fujimori, Hiroshi*; Umegaki, Izumi*; Nakamura, Jumpei*; Fujihara, Masayoshi; Tampo, Motonobu*; Kawamura, Naritoshi*; et al.
Interactions (Internet), 245(1), p.31_1 - 31_6, 2024/12
Kato, Yuto*; Sasaki, Takayuki*; Tonna, Ryutaro*; Kobayashi, Taishi*; Okamoto, Yoshihiro
Applied Geochemistry, 175, p.106196_1 - 106196_9, 2024/11
Times Cited Count:1 Percentile:0.00(Geochemistry & Geophysics)Kobayashi, Taishi*; Sato, Yutaro*; Tonna, Ryutaro*; Matsumura, Daiju; Sasaki, Takayuki*; Ikeda, Atsushi
Dalton Transactions (Internet), 53(46), p.18616 - 18628, 2024/10
Times Cited Count:0 Percentile:0.00(Chemistry, Inorganic & Nuclear)Endo, Shunsuke; Abe, Ryota*; Fujioka, Hiroyuki*; Ino, Takashi*; Iwamoto, Osamu; Iwamoto, Nobuyuki; Kawamura, Shiori*; Kimura, Atsushi; Kitaguchi, Masaaki*; Kobayashi, Ryuju*; et al.
European Physical Journal A, 60(8), p.166_1 - 166_10, 2024/08
Times Cited Count:2 Percentile:78.45(Physics, Nuclear) -odd/
-odd/ -odd interactions on the 0.75 eV
-odd interactions on the 0.75 eV  -wave resonance in
-wave resonance in 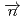 +
+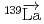 forward transmission determined using a pulsed neutron beam
forward transmission determined using a pulsed neutron beamNakabe, Rintaro*; Auton, C. J.*; Endo, Shunsuke; Fujioka, Hiroyuki*; Gudkov, V.*; Hirota, Katsuya*; Ide, Ikuo*; Ino, Takashi*; Ishikado, Motoyuki*; Kambara, Wataru*; et al.
Physical Review C, 109(4), p.L041602_1 - L041602_4, 2024/04
Times Cited Count:1 Percentile:23.17(Physics, Nuclear) -wave resonance of
-wave resonance of 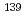
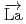 +
+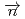
Okudaira, Takuya*; Nakabe, Rintaro*; Auton, C. J.*; Endo, Shunsuke; Fujioka, Hiroyuki*; Gudkov, V.*; Ide, Ikuo*; Ino, Takashi*; Ishikado, Motoyuki*; Kambara, Wataru*; et al.
Physical Review C, 109(4), p.044606_1 - 044606_9, 2024/04
Times Cited Count:2 Percentile:78.45(Physics, Nuclear)
Linh, B. D.*; Corsi, A.*; Gillibert, A.*; Obertelli, A.*; Doornenbal, P.*; Barbieri, C.*; Duguet, T.*; G mez-Ramos, M.*; Holt, J. D.*; Hu, B. S.*; et al.
mez-Ramos, M.*; Holt, J. D.*; Hu, B. S.*; et al.
Physical Review C, 109(3), p.034312_1 - 034312_15, 2024/03
Times Cited Count:2 Percentile:58.81(Physics, Nuclear)no abstracts in English
Tonna, Ryutaro*; Sasaki, Takayuki*; Okamoto, Yoshihiro; Kobayashi, Taishi*; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*
Journal of Nuclear Materials, 589, p.154862_1 - 154862_10, 2024/02
Times Cited Count:2 Percentile:46.61(Materials Science, Multidisciplinary)The dissolution behavior of FeUO compounds formed by a high-temperature reaction of UO
compounds formed by a high-temperature reaction of UO with iron, a stainless-steel component of reactor structural materials, was investigated under atmospheric conditions. The compounds were prepared in an electric furnace using U
with iron, a stainless-steel component of reactor structural materials, was investigated under atmospheric conditions. The compounds were prepared in an electric furnace using U O
O and Fe
and Fe O
O as starting materials, and their solid states were analyzed using X-ray diffraction, scanning electron microscopy energy dispersive X-ray spectroscopy, and X-ray absorption fine structure spectroscopy. The concentration of nuclides dissolved in water was examined by performing static leaching tests of FeUO
as starting materials, and their solid states were analyzed using X-ray diffraction, scanning electron microscopy energy dispersive X-ray spectroscopy, and X-ray absorption fine structure spectroscopy. The concentration of nuclides dissolved in water was examined by performing static leaching tests of FeUO compounds for up to three months. A redox reaction was proposed to occur between trivalent Fe and pentavalent U ions in the early stage of FeUO
compounds for up to three months. A redox reaction was proposed to occur between trivalent Fe and pentavalent U ions in the early stage of FeUO dissolution. It was thermodynamically deduced that the reduced divalent Fe ion was finally oxidized into a trivalent ion in the presence of dissolved oxygen, and iron hydroxide limited the solubility of Fe. Meanwhile, the concentration of hexavalent U (i.e., uranyl ion) was limited owing to the presence of secondary minerals such as metaschoepite and sodium uranate and subsequently decreased, possibly owing to sorption on Fe oxides, for example. The concentrations of multivalent ions of fission products, such as Ru and Ce, also decreased, likely for the reason above. By contrast, the concentration of soluble Cs ions did not decrease. The validity of this interpretation was supported by comparing the results with the dissolution behavior of a reference sample (Fe-free U
dissolution. It was thermodynamically deduced that the reduced divalent Fe ion was finally oxidized into a trivalent ion in the presence of dissolved oxygen, and iron hydroxide limited the solubility of Fe. Meanwhile, the concentration of hexavalent U (i.e., uranyl ion) was limited owing to the presence of secondary minerals such as metaschoepite and sodium uranate and subsequently decreased, possibly owing to sorption on Fe oxides, for example. The concentrations of multivalent ions of fission products, such as Ru and Ce, also decreased, likely for the reason above. By contrast, the concentration of soluble Cs ions did not decrease. The validity of this interpretation was supported by comparing the results with the dissolution behavior of a reference sample (Fe-free U O
O ).
).
 , Zr, and stainless steel and leaching behavior of the fission products and matrix elements
, Zr, and stainless steel and leaching behavior of the fission products and matrix elementsTonna, Ryutaro*; Sasaki, Takayuki*; Kodama, Yuji*; Kobayashi, Taishi*; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Kumagai, Yuta; Kusaka, Ryoji; Watanabe, Masayuki
Nuclear Engineering and Technology, 55(4), p.1300 - 1309, 2023/04
Times Cited Count:6 Percentile:80.64(Nuclear Science & Technology)Simulated debris was synthesized using UO , Zr, and stainless steel and a heat treatment method under inert or oxidizing conditions. The primary U solid phase of the debris synthesized at 1473 K under inert conditions was UO
, Zr, and stainless steel and a heat treatment method under inert or oxidizing conditions. The primary U solid phase of the debris synthesized at 1473 K under inert conditions was UO , whereas a (U,Zr)O
, whereas a (U,Zr)O solid solution formed at 1873 K. Under oxidizing conditions, a mixture of U
solid solution formed at 1873 K. Under oxidizing conditions, a mixture of U O
O and (Fe,Cr)UO
and (Fe,Cr)UO phases formed at 1473 K whereas a (U,Zr)O
phases formed at 1473 K whereas a (U,Zr)O solid solution formed at 1873 K. The leaching behavior of the fission products from the simulated debris was evaluated using two methods: the irradiation method, for which fission products were produced via neutron irradiation, and the doping method, for which trace amounts of non-radioactive elements were doped into the debris. The dissolution behavior of U depended on the properties of the debris and aqueous medium the debris was immersed in. Cs, Sr, and Ba leached out regardless of the primary solid phases. The leaching of high-valence Eu and Ru ions was suppressed, possibly owing to their solid-solution reaction with or incorporation into the uranium compounds of the simulated debris.
solid solution formed at 1873 K. The leaching behavior of the fission products from the simulated debris was evaluated using two methods: the irradiation method, for which fission products were produced via neutron irradiation, and the doping method, for which trace amounts of non-radioactive elements were doped into the debris. The dissolution behavior of U depended on the properties of the debris and aqueous medium the debris was immersed in. Cs, Sr, and Ba leached out regardless of the primary solid phases. The leaching of high-valence Eu and Ru ions was suppressed, possibly owing to their solid-solution reaction with or incorporation into the uranium compounds of the simulated debris.
Shimomura, Koichiro*; Koda, Akihiro*; Pant, A. D.*; Natori, Hiroaki*; Fujimori, Hiroshi*; Umegaki, Izumi*; Nakamura, Jumpei*; Tampo, Motonobu*; Kawamura, Naritoshi*; Teshima, Natsuki*; et al.
Journal of Physics; Conference Series, 2462, p.012033_1 - 012033_5, 2023/03
Times Cited Count:0 Percentile:0.00(Physics, Applied)Kobayashi, Taishi*; Fushimi, Tomokazu*; Mizukoshi, Hirofumi*; Motokawa, Ryuhei; Sasaki, Takayuki*
Langmuir, 38(48), p.14656 - 14665, 2022/12
Times Cited Count:3 Percentile:21.39(Chemistry, Multidisciplinary)Okamoto, Yoshihiro; Shiwaku, Hideaki; Shimamura, Keisuke*; Kobayashi, Hidekazu; Nagai, Takayuki; Inose, Takehiko*; Sato, Seiichi*; Hatakeyama, Kiyoshi*
Journal of Nuclear Materials, 570, p.153962_1 - 153962_13, 2022/11
Simulated nuclear waste glass samples containing phosphorus, which increase the solubility of molybdenum, were prepared and analyzed using synchrotron X-ray Absorption Fine Structure (XAFS) analysis for some constituent elements and Raman spectroscopic analysis of their complex structure. Changes in local structure and chemical state due to different phosphorus additions and waste loading rates were systematically studied. Consequently, no crystalline phase due to the molybdate compound was observed even at a maximum waste content of 30 wt% (corresponding to 1.87 mol% MoO ). Oxidation proceeded when the waste-loading rate was increased, whereas the reduction proceeded when phosphorus was added. In some cases, the effects of oxidation and reduction were offset. The local structure around specific elements can be classified as follows; Zn that is affected mainly by the waste-loading rate, Ce that is affected by both the waste-loading rate and phosphorus addition, and Zr element that is not affected by either of them. From the comparison between the analytical results of Mo and other elements, it was considered that the added phosphorus exists as a free PO
). Oxidation proceeded when the waste-loading rate was increased, whereas the reduction proceeded when phosphorus was added. In some cases, the effects of oxidation and reduction were offset. The local structure around specific elements can be classified as follows; Zn that is affected mainly by the waste-loading rate, Ce that is affected by both the waste-loading rate and phosphorus addition, and Zr element that is not affected by either of them. From the comparison between the analytical results of Mo and other elements, it was considered that the added phosphorus exists as a free PO structural unit and may deprive the alkali metal coordinated to the molybdate ion.
structural unit and may deprive the alkali metal coordinated to the molybdate ion.
Kato, Masato; Machida, Masahiko; Hirooka, Shun; Nakamichi, Shinya; Ikusawa, Yoshihisa; Nakamura, Hiroki; Kobayashi, Keita; Ozawa, Takayuki; Maeda, Koji; Sasaki, Shinji; et al.
Materials Science and Fuel Technologies of Uranium and Plutonium mixed Oxide, 171 Pages, 2022/10
Innovative and advanced nuclear reactors using plutonium fuel has been developed in each country. In order to develop a new nuclear fuel, irradiation tests are indispensable, and it is necessary to demonstrate the performance and safety of nuclear fuels. If we can develop a technology that accurately simulates irradiation behavior as a technology that complements the irradiation test, the cost, time, and labor involved in nuclear fuel research and development will be greatly reduced. And safety and reliability can be significantly improved through simulation of nuclear fuel irradiation behavior. In order to evaluate the performance of nuclear fuel, it is necessary to know the physical and chemical properties of the fuel at high temperatures. And it is indispensable to develop a behavior model that describes various phenomena that occur during irradiation. In previous research and development, empirical methods with fitting parameters have been used in many parts of model development. However, empirical techniques can give very different results in areas where there is no data. Therefore, the purpose of this study is to construct a scientific descriptive model that can extrapolate the basic characteristics of fuel to the composition and temperature, and to develop an irradiation behavior analysis code to which the model is applied.
Ohshima, Hiroyuki; Morishita, Masaki*; Aizawa, Kosuke; Ando, Masanori; Ashida, Takashi; Chikazawa, Yoshitaka; Doda, Norihiro; Enuma, Yasuhiro; Ezure, Toshiki; Fukano, Yoshitaka; et al.
Sodium-cooled Fast Reactors; JSME Series in Thermal and Nuclear Power Generation, Vol.3, 631 Pages, 2022/07
This book is a collection of the past experience of design, construction, and operation of two reactors, the latest knowledge and technology for SFR designs, and the future prospects of SFR development in Japan. It is intended to provide the perspective and the relevant knowledge to enable readers to become more familiar with SFR technology.
 and Eu
and Eu ions onto sedimentary rock in the presence of gamma-irradiated humic acid
ions onto sedimentary rock in the presence of gamma-irradiated humic acidZhao, Q.*; Saito, Takeshi*; Miyakawa, Kazuya; Sasamoto, Hiroshi; Kobayashi, Taishi*; Sasaki, Takayuki*
Journal of Hazardous Materials, 428, p.128211_1 - 128211_10, 2022/04
Times Cited Count:7 Percentile:47.21(Engineering, Environmental)The influence of humic acid and its radiological degradation on the sorption of Cs and Eu
and Eu by sedimentary rock was investigated to understand the sorption process of metal ions and humic substances. Aldrich humic acid (HA) solution was irradiated with different doses of gamma irradiation using a Co-60 gamma-ray source prior to the contact between the metal ions and the solid sorbent. The HA molecule decomposed to smaller molecules with a lower complexation affinity. Batch sorption experiments were performed to evaluate the effect of gamma-irradiated HA on the sorption of Cs
by sedimentary rock was investigated to understand the sorption process of metal ions and humic substances. Aldrich humic acid (HA) solution was irradiated with different doses of gamma irradiation using a Co-60 gamma-ray source prior to the contact between the metal ions and the solid sorbent. The HA molecule decomposed to smaller molecules with a lower complexation affinity. Batch sorption experiments were performed to evaluate the effect of gamma-irradiated HA on the sorption of Cs and Eu
and Eu ions. The addition of non-irradiated HA weakened the sorption of Eu because of the lower sorption of the neutral or negatively charged Eu-HA complexes compared with free Eu ions. The sorption of monovalent Cs ions was barely affected by the presence of HA and its gamma irradiation. The concentration ratio of HA complexed species and non-complexed species in the solid and liquid phases was evaluated by sequential filtration and chemical equilibrium calculations. The ratios supported the minimal contribution of HA to Cs sorption. However, the concentration ratio for Eu
ions. The addition of non-irradiated HA weakened the sorption of Eu because of the lower sorption of the neutral or negatively charged Eu-HA complexes compared with free Eu ions. The sorption of monovalent Cs ions was barely affected by the presence of HA and its gamma irradiation. The concentration ratio of HA complexed species and non-complexed species in the solid and liquid phases was evaluated by sequential filtration and chemical equilibrium calculations. The ratios supported the minimal contribution of HA to Cs sorption. However, the concentration ratio for Eu in the liquid phase was high, indicating that the complexing ability of HA to Eu
in the liquid phase was high, indicating that the complexing ability of HA to Eu was higher than that of HA to Cs
was higher than that of HA to Cs ions. Therefore, the sorption of free Eu
ions. Therefore, the sorption of free Eu would predominate with the gamma irradiation dose applied to the HA solution under a radiation field near the HLW package.
would predominate with the gamma irradiation dose applied to the HA solution under a radiation field near the HLW package.
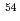 Ca; Spectroscopy of
Ca; Spectroscopy of 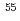 K,
K, 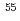 Ca, and
Ca, and 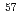 Ca
CaKoiwai, Takuma*; Wimmer, K.*; Doornenbal, P.*; Obertelli, A.*; Barbieri, C.*; Duguet, T.*; Holt, J. D.*; Miyagi, Takayuki*; Navr til, P.*; Ogata, Kazuyuki*; et al.
til, P.*; Ogata, Kazuyuki*; et al.
Physics Letters B, 827, p.136953_1 - 136953_7, 2022/04
Times Cited Count:7 Percentile:67.20(Astronomy & Astrophysics)no abstracts in English
Motoshima, Takayuki*; Matsui, Hiroya; Kawakubo, Masahiro*; Kobayashi, Masato*; Ichimura, Tetsuhiro*; Sugita, Yutaka
Nihon Genshiryoku Gakkai-Shi ATOMO , 64(3), p.163 - 167, 2022/03
, 64(3), p.163 - 167, 2022/03
no abstracts in English
Kimata, Tetsuya*; Kakitani, Kenta*; Yamamoto, Shunya*; Shimoyama, Iwao; Matsumura, Daiju; Iwase, Akihiro*; Mao, W.*; Kobayashi, Tomohiro*; Yamaki, Tetsuya*; Terai, Takayuki*
Physical Review Materials (Internet), 6(3), p.035801_1 - 035801_7, 2022/03
Times Cited Count:7 Percentile:46.06(Materials Science, Multidisciplinary)Sato, Nobuaki*; Kirishima, Akira*; Watanabe, Masayuki; Sasaki, Takayuki*; Uehara, Akihiro*; Takeda, Shino*; Kitatsuji, Yoshihiro; Otobe, Haruyoshi; Kobayashi, Taishi*
The Chemistry of Thorium, Plutonium and MA, 254 Pages, 2022/03
The chemistry of nuclear materials such as Thorium (Part 1) and Plutonium (Part 2) was described in relation from the fundamentals on solid chemistry and solution chemistry to the practicals on the experiment and evaluation method in detail. Minor actinides such as Neptunium, Americium, Curium and Protoactinium, was introduced the basics on the solid and solution chemistry.