Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tanaka, Nobuyuki; Sawada, Shinichi*; Yamaki, Tetsuya*; Kodaira, Takahide*; Kimura, Takehiro*; Nomura, Mikihiro*
Chemical Engineering Science, 237, p.116575_1 - 116575_11, 2021/06
Times Cited Count:3 Percentile:11.41(Engineering, Chemical)We have been developing the ion exchange membranes by a radiation grafted polymerization method to improve HI concentration performance for Electro-electrodialysis (EED) in the thermochemical water-splitting hydrogen production iodine-sulfur process. In this work, the crosslinking structures were introduced to the ion exchange membranes. The proton conductivity ( ), transport number (t
), transport number (t ), and water permeation factor (
), and water permeation factor ( ) of these crosslinked ion exchange membranes were measured and the effect of crosslinks to these performance indexes were investigated. The introduction of crosslinks was found to improve the selectivity of H
) of these crosslinked ion exchange membranes were measured and the effect of crosslinks to these performance indexes were investigated. The introduction of crosslinks was found to improve the selectivity of H and water transport (increase of t
and water transport (increase of t and decrease of
and decrease of  ), although the
), although the  somewhat decreased. The EED model that we established to discuss the permeation mechanism of EED system was used to theoretically analyze the effect of crosslink on the performance indexes. Based on this analysis of measurement results, the introduction of the crosslink was found to little affect the absorbed amount of HIx solution and H
somewhat decreased. The EED model that we established to discuss the permeation mechanism of EED system was used to theoretically analyze the effect of crosslink on the performance indexes. Based on this analysis of measurement results, the introduction of the crosslink was found to little affect the absorbed amount of HIx solution and H diffusion coefficient in the tested membranes, whereas it could lead to decrease I
diffusion coefficient in the tested membranes, whereas it could lead to decrease I diffusion coefficient. The results of
diffusion coefficient. The results of  and t
and t could reflect these effects. In addition, we found the fact that crosslink can inhibit the swelling due to the absorption of the HIx solution. As a result, the
could reflect these effects. In addition, we found the fact that crosslink can inhibit the swelling due to the absorption of the HIx solution. As a result, the  value decreased owing to the introduction of crosslink.
value decreased owing to the introduction of crosslink.
Sawada, Shinichi*; Kimura, Takehiro*; Nishijima, Haruyuki*; Kodaira, Takahide*; Tanaka, Nobuyuki; Kubo, Shinji; Imabayashi, Shinichiro*; Nomura, Mikihiro*; Yamaki, Tetsuya*
International Journal of Hydrogen Energy, 45(27), p.13814 - 13820, 2020/05
Times Cited Count:2 Percentile:4.88(Chemistry, Physical)An electrochemical membrane Bunsen reaction using a cation exchange membrane (CEM) is a key to achieving an iodine-sulfur (IS) thermochemical water splitting process for mass-production of hydrogen. In this study, we prepared both the radiation-grafted CEM with a high ion exchange capacity (IEC) and the highly-porous Au-electroplated anode, and then used them for the membrane Bunsen reaction to reduce the cell overvoltage. The high-IEC grafted CEM exhibited low resistivity for proton transport, while the porous Au anode had a large effective surface area for anodic SO oxidation reaction. As a result, the cell overvoltage for the membrane Bunsen reaction was significantly reduced to 0.21 V at 200 mA/cm
oxidation reaction. As a result, the cell overvoltage for the membrane Bunsen reaction was significantly reduced to 0.21 V at 200 mA/cm , which was only one-third of that of the previous test using the commercial CEM and non-porous anode. From the analysis of the current-voltage characteristics, employment of the grafted CEM was found to be more effective for the overvoltage reduction compared to the porous Au anode.
, which was only one-third of that of the previous test using the commercial CEM and non-porous anode. From the analysis of the current-voltage characteristics, employment of the grafted CEM was found to be more effective for the overvoltage reduction compared to the porous Au anode.
Nomura, Mikihiro*; Kodaira, Takahide*; Ikeda, Ayumi*; Naka, Yasuhito*; Nishijima, Haruyuki*; Imabayashi, Shinichiro*; Sawada, Shinichi*; Yamaki, Tetsuya*; Tanaka, Nobuyuki; Kubo, Shinji
Journal of Chemical Engineering of Japan, 51(9), p.726 - 731, 2018/09
Times Cited Count:3 Percentile:11.27(Engineering, Chemical)Thermochemical hydrogen production by the iodine-sulfur process decomposes water into hydrogen and oxygen by combining the chemical reactions of iodine and sulfur. Two types of acids are produced through the Bunsen reaction. To improve the performance of this reaction, ion-exchange membranes for the membrane Bunsen reaction should be developed. In the present study, a cation-exchange membrane was prepared by using a radiation-graft polymerization method. It was found that a divinylbenzene crosslinking procedure was very effective in reducing water permeation through the membrane, and the membrane Bunsen reaction was successfully carried out by using the developed crosslinked membrane. Therefore, the developed crosslinked membrane is a potential candidate for cation-exchange membranes for the membrane Bunsen reaction.
Kodaira, Takahide; Ikeda, Ayumi*; Matsuyama, Emi*; Kono, Nobuho*; Sawada, Shinichi; Yamaki, Tetsuya; Nomura, Mikihiro*
no journal, ,
In the thermochemical water splitting IS process, the Bunsen reaction (SO + I
+ I + 2H
+ 2H O = H
O = H SO
SO + 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane, which renders separation procedures unnecessary. As part of a JST-ALCA project, therefore, we have been developing ion exchange membranes for this application by using methods of radiation crosslinking and/or radiation graft polymerization. Our preliminary experiments made it possible to confirm controllability of the degree of grafting,
+ 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane, which renders separation procedures unnecessary. As part of a JST-ALCA project, therefore, we have been developing ion exchange membranes for this application by using methods of radiation crosslinking and/or radiation graft polymerization. Our preliminary experiments made it possible to confirm controllability of the degree of grafting, 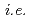 , ion exchange capacity by varying the conditions of the grafting. Importantly, controlling the fixed-charge density of the membrane should both lower permeability of SO
, ion exchange capacity by varying the conditions of the grafting. Importantly, controlling the fixed-charge density of the membrane should both lower permeability of SO and enhance the transport number of H
and enhance the transport number of H .
.
Kodaira, Takahide*; Ikeda, Ayumi*; Matsuyama, Emi*; Kono, Nobuho*; Oura, Kotone*; Sawada, Shinichi; Yamaki, Tetsuya; Nomura, Mikihiro*
no journal, ,
There has been a strong motivation to develop new cation exchange membranes suitable for the Bunsen reaction in the thermochemical water splitting IS process. We prepared cation exchange membranes by a radiation grafting polymerization method. The grafting reaction into a poly(ethylene-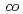 -tetrafluoroethylene) film was performed in a mixture of styrene and divinylbenzene (DVB). The membrane had an ion exchange capacity of 2.17 mmol g
-tetrafluoroethylene) film was performed in a mixture of styrene and divinylbenzene (DVB). The membrane had an ion exchange capacity of 2.17 mmol g while Nafion212 possessed 0.90 mmol g
while Nafion212 possessed 0.90 mmol g that is less than half of that for the grafted membrane. Both these membranes exhibited a similar water uptake, but the grafted membrane showed lower water permeation than Nafion212.
that is less than half of that for the grafted membrane. Both these membranes exhibited a similar water uptake, but the grafted membrane showed lower water permeation than Nafion212.
Kodaira, Takahide*; Ikeda, Ayumi*; Matsuyama, Emi*; Kono, Nobuho*; Oura, Kotone*; Sawada, Shinichi; Yamaki, Tetsuya; Nomura, Mikihiro*
no journal, ,
In the thermochemical water splitting IS process, the Bunsen reaction (SO + I
+ I + 2H
+ 2H O = H
O = H SO
SO + 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane, which renders separation procedures unnecessary. As part of a JST-ALCA project, therefore, we have been developing ion exchange membranes for this application by radiation-induced graft polymerization. The water permeation of the grafted membrane was lower than that of Nafion 212 under the condition of a similar water uptake.
+ 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane, which renders separation procedures unnecessary. As part of a JST-ALCA project, therefore, we have been developing ion exchange membranes for this application by radiation-induced graft polymerization. The water permeation of the grafted membrane was lower than that of Nafion 212 under the condition of a similar water uptake.
Kodaira, Takahide*; Ikeda, Ayumi*; Matsuyama, Emi*; Kono, Nobuho*; Oura, Kotone*; Sawada, Shinichi; Yamaki, Tetsuya; Nomura, Mikihiro*
no journal, ,
In the thermochemical water splitting IS process, the Bunsen reaction (SO + I
+ I + 2H
+ 2H O = H
O = H SO
SO + 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane. As part of an ongoing JST-ALCA project, therefore, we developed ion exchange membranes for this reaction by radiation-induced graft polymerization, investigating their water permeation properties by the pervaporation method. Our membrane preparation involves the
+ 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane. As part of an ongoing JST-ALCA project, therefore, we developed ion exchange membranes for this reaction by radiation-induced graft polymerization, investigating their water permeation properties by the pervaporation method. Our membrane preparation involves the  -ray-induced grafting of styrene and divinylbenzene into poly(ethylene-
-ray-induced grafting of styrene and divinylbenzene into poly(ethylene-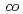 -tetrafluoroethylene) films and the subsequent sulfonation. Water permeate flux values at 25
-tetrafluoroethylene) films and the subsequent sulfonation. Water permeate flux values at 25 C were 7.4 and 19 kg/m
C were 7.4 and 19 kg/m h through the grafted membrane and Nafion 212 at the same water uptake (37%), respectively.
h through the grafted membrane and Nafion 212 at the same water uptake (37%), respectively.
Kodaira, Takahide*; Ikeda, Ayumi*; Oura, Kotone*; Ono, Ryuhei*; Matsuyama, Emi*; Sawada, Shinichi; Yamaki, Tetsuya; Nomura, Mikihiro*
no journal, ,
no abstracts in English
Kodaira, Takahide*; Ikeda, Ayumi*; Matsuyama, Emi*; Oura, Kotone*; Sawada, Shinichi; Yamaki, Tetsuya; Nomura, Mikihiro*
no journal, ,
There has been a strong motivation to develop new cation exchange membranes suitable for the Bunsen reaction in the thermochemical water splitting IS process. We prepared cation exchange membranes by a radiation grafting polymerization method. The grafting reaction into a poly(ethylene-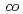 -tetrafluoroethylene) film was performed in a mixture of styrene and divinylbenzene (DVB). The grafted membrane showed two times lower water permeation flux and three times higher activation energy of water diffusion than Nafion212 though both the membranes exhibited a similar water uptake. Therefore, the DVB-based crosslinking in the graft polymer would restrict water permeation through the membrane.
-tetrafluoroethylene) film was performed in a mixture of styrene and divinylbenzene (DVB). The grafted membrane showed two times lower water permeation flux and three times higher activation energy of water diffusion than Nafion212 though both the membranes exhibited a similar water uptake. Therefore, the DVB-based crosslinking in the graft polymer would restrict water permeation through the membrane.
Kodaira, Takahide*; Oura, Kotone*; Ikeda, Ayumi*; Ono, Ryuhei*; Matsuyama, Emi*; Nomura, Mikihiro*; Sawada, Shinichi; Yamaki, Tetsuya; Tanaka, Nobuyuki; Kubo, Shinji
no journal, ,
Bunsen reactor in the IS process has a potential of the downsizing and the improvement of efficiency by using redox type reactor with an ion exchange membrane. The key to the performance of redox reactor is the development of the high performance ion exchange membrane. In this paper, we investigated the performance (proton transport number (t ) and water permeation factor (
) and water permeation factor ( )) of Nafion 212, which is the reference. As a result, t
)) of Nafion 212, which is the reference. As a result, t and
and  were 0.63 and 2.82, respectively, indicating that not only H
were 0.63 and 2.82, respectively, indicating that not only H but also I
but also I and water permeate through the membrane. The permeation of these components might cause the precipitation of sulfur and the rising of the voltage. Aftertime, we must new ion exchange membrane which can restrict the permeation of I
and water permeate through the membrane. The permeation of these components might cause the precipitation of sulfur and the rising of the voltage. Aftertime, we must new ion exchange membrane which can restrict the permeation of I and water.
and water.
Tanaka, Nobuyuki; Sawada, Shinichi*; Yamaki, Tetsuya*; Kodaira, Takahide*; Kimura, Takehiro*; Nomura, Mikihiro*
no journal, ,
JAEA has been conducted R&D on thermochemical water-splitting Iodine-Sulfur (IS) process. IS process has hydrogen iodide (HI) concentration section by electro-membrane process, which is so-called the electro-electrodialysis (EED). EED is the key unit operation to improve the process efficiency. In our previous study, the cation exchange membrane (CEM) was prepared by radiation-induced grafting method, and applied to EED. Our previous study suggested that permeation of anionic species and water must be inhibited to increase H selectivity. In the present study, to achieve this requirement, we used the novel crosslinked grafted CEMs, which were prepared by co-grafting of crosslinked agent. The performance was also evaluated experimentally for the conventional non-crosslinked and crosslinked CEMs by carrying out HI concentration tests. In conclusion, due to the cross-linking structure, we found the improvement of concentration performance.
selectivity. In the present study, to achieve this requirement, we used the novel crosslinked grafted CEMs, which were prepared by co-grafting of crosslinked agent. The performance was also evaluated experimentally for the conventional non-crosslinked and crosslinked CEMs by carrying out HI concentration tests. In conclusion, due to the cross-linking structure, we found the improvement of concentration performance.
Kodaira, Takahide; Ikeda, Ayumi*; Matsuyama, Emi*; Kono, Nobuho*; Sawada, Shinichi; Yamaki, Tetsuya; Nomura, Mikihiro*
no journal, ,
In the thermochemical water splitting IS process, the Bunsen reaction (SO + I
+ I + 2H
+ 2H O = H
O = H SO
SO + 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane, which renders separation procedures unnecessary. As part of a JST-ALCA project, therefore, we have been developing ion exchange membranes for this application by using methods of radiation crosslinking and/or radiation graft polymerization. Our preliminary experiments made it possible to confirm controllability of the degree of grafting,
+ 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane, which renders separation procedures unnecessary. As part of a JST-ALCA project, therefore, we have been developing ion exchange membranes for this application by using methods of radiation crosslinking and/or radiation graft polymerization. Our preliminary experiments made it possible to confirm controllability of the degree of grafting, 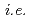 , ion exchange capacity by varying the conditions of the grafting.
, ion exchange capacity by varying the conditions of the grafting.