Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...

 values calculated from radiocesium interception potential
values calculated from radiocesium interception potentialUno, Koichiro*; Nakao, Atsushi*; Okumura, Masahiko; Yamaguchi, Akiko; Kogure, Toshihiro*; Yanai, Junta*
Nihon Dojo Hiryo Gaku Zasshi, 94(5), p.376 - 384, 2023/10
Radiocesium interception potential (RIP) has been widely used as a quantitative indicator of cesium (Cs) adsorption capacity of soil, but it has been found that RIP does not always correlate with the distribution coefficient (
 ) of Cs in the actual environment. In order to clarify the cause of this discrepancy, we measured Kd using more realistic solutions, compared it with RIP, and evaluated the mineral structure. As a result, it was found that the concentration of competing cations, such as potassium and ammonium ions, and the structural change of the mineral itself are important.
) of Cs in the actual environment. In order to clarify the cause of this discrepancy, we measured Kd using more realistic solutions, compared it with RIP, and evaluated the mineral structure. As a result, it was found that the concentration of competing cations, such as potassium and ammonium ions, and the structural change of the mineral itself are important.
Igarashi, Yasuhito*; Kogure, Toshihiro*; Kuribara, Yuichi; Miura, Hikaru*; Okumura, Taiga*; Satou, Yukihiko; Takahashi, Yoshio*; Yamaguchi, Noriko*
Journal of Environmental Radioactivity, 205-206, p.101 - 118, 2019/09
Times Cited Count:82 Percentile:68.16(Environmental Sciences)Scientists face challenge in identifying the radioactive materials which are found as dotted images on various imaging plate (IP) autoradiographic photos of radioactively contaminated materials by the Fukushima Dai-ichi Nuclear Power Plant (F1NPP, or FDNPP) accident, such as air filter, fugitive dust, surface soil, agricultural materials, and water-shed samples. It has been revealed that they are minute particles with distinct morphology and elemental composition with high specific radioactivity, and different from those of the so-called Chernobyl hot particles. Basically, they are glassy particles once molten, composed of Si, O, Fe, Zn etc. with highly concentrated radiocaesium, which can be called as radiocaesium-bearing microparticles (CsMP). At present, CsMP can be classified into two types, Types-A and -B, which are characterized by different specific radioactivity,  Cs/
Cs/ Cs ratio, size and morphology, and geographic distribution around F1NPP. Such studies on the CsMP from various aspects have provided valuable information about what happened in the nuclear reactors during the F1NPP accident and fates of the CsMP in the environment. This review first provides a retrospective view on the research history of the CsMP, which is helpful to understand the unique character of the CsMP. Subsequently, more details about the current understanding of the natures of these hot particles, such as origin, morphology, chemical compositions, thermal properties, water-solubility, and secondary migration of CsMP in river and ocean systems are described with future prospects.
Cs ratio, size and morphology, and geographic distribution around F1NPP. Such studies on the CsMP from various aspects have provided valuable information about what happened in the nuclear reactors during the F1NPP accident and fates of the CsMP in the environment. This review first provides a retrospective view on the research history of the CsMP, which is helpful to understand the unique character of the CsMP. Subsequently, more details about the current understanding of the natures of these hot particles, such as origin, morphology, chemical compositions, thermal properties, water-solubility, and secondary migration of CsMP in river and ocean systems are described with future prospects.
Okumura, Taiga*; Yamaguchi, Noriko*; Dohi, Terumi; Iijima, Kazuki; Kogure, Toshihiro*
Microscopy, 68(3), p.234 - 242, 2019/06
Times Cited Count:11 Percentile:62.64(Microscopy)Radiocesium-bearing microparticles (CsMPs), consisting substantially of silicate glass, were released to the environment during the Fukushima nuclear accident in March 2011. We investigated a total of nine CsMPs using transmission electron microscopy (TEM) and inferred the atmosphere in the reactors during the accident. From elemental mapping using energy-dispersive X-ray spectrometry, Fe and Zn showing radial inhomogeneities were found in the CsMPs, in addition to the Cs that had been previously reported. Four of the CsMPs included submicron crystals, which were identified as chromite, franklinite, acanthite, molybdenite, and hessite. The chromium-containing spinels, chromite and franklinite, indicated the presence of ferrous iron (Fe ), suggesting that the inside of the reactors was reductive to some extent. Electron energy-loss spectroscopy also confirmed that the CsMPs did not contain boron, and therefore the atmosphere in which they were formed might be boron-free.
), suggesting that the inside of the reactors was reductive to some extent. Electron energy-loss spectroscopy also confirmed that the CsMPs did not contain boron, and therefore the atmosphere in which they were formed might be boron-free.
Okumura, Taiga*; Yamaguchi, Noriko*; Dohi, Terumi; Iijima, Kazuki; Kogure, Toshihiro*
Scientific Reports (Internet), 9(1), p.3520_1 - 3520_9, 2019/03
Times Cited Count:42 Percentile:87.58(Multidisciplinary Sciences)Radiocesium-bearing microparticles (CsMPs) were released by the FDNPP accident. We conducted dissolution experiments of CsMPs by reaction with pure water absorbing CO  from the atmosphere and seawater. The activation energy for the dissolution of CsMPs was estimated to be 67 and 89 kJ/mol, and the dissolution rate at 13
from the atmosphere and seawater. The activation energy for the dissolution of CsMPs was estimated to be 67 and 89 kJ/mol, and the dissolution rate at 13 C was 0.011 and 0.130
C was 0.011 and 0.130  m/y for pure water and seawater, respectively. Probably the faster dissolution rate in seawater than in pure water is mainly owing to the difference in pH. The shapes of CsMPs dissolved in pure water were considerably altered. Tin oxide and iron oxide nanoparticulates were formed on their surfaces. Such features were similar to those observed in a CsMP collected recently in Fukushima Prefecture, indicating that dissolution of CsMPs is also occurring in the environment. In the case of CsMPs dissolved in seawater, a crust of secondary minerals rich in Mg and Fe was formed and the glass matrix became smaller inside the crust.
m/y for pure water and seawater, respectively. Probably the faster dissolution rate in seawater than in pure water is mainly owing to the difference in pH. The shapes of CsMPs dissolved in pure water were considerably altered. Tin oxide and iron oxide nanoparticulates were formed on their surfaces. Such features were similar to those observed in a CsMP collected recently in Fukushima Prefecture, indicating that dissolution of CsMPs is also occurring in the environment. In the case of CsMPs dissolved in seawater, a crust of secondary minerals rich in Mg and Fe was formed and the glass matrix became smaller inside the crust.
Nihei, Naoto*; Yoshimura, Kazuya; Okumura, Taiga*; Tanoi, Keitaro*; Iijima, Kazuki; Kogure, Toshihiro*; Nakanishi, Tomoko*
Journal of Radioanalytical and Nuclear Chemistry, 318(1), p.341 - 346, 2018/10
Times Cited Count:5 Percentile:39.83(Chemistry, Analytical)Okumura, Taiga*; Yamaguchi, Noriko*; Dohi, Terumi; Iijima, Kazuki; Kogure, Toshihiro*
Scientific Reports (Internet), 8, p.9707_1 - 9707_8, 2018/06
Times Cited Count:12 Percentile:34.44(Multidisciplinary Sciences)Radiocesium-bearing microparticles (CsP) substantially made of silicate glass are a novel form of radiocesium (RCs) emitted from the Fukushima Dai-ichi NPP. CsPs have a potential risk of internal radiation exposure caused by inhalation. Radiation-contaminated waste (Rcw) including CsPs is being burned in incinerators; therefore, this study has investigated the responses of CsPs to heating in air. The radioactivity of CsPs gradually decreased from 600  C and was almost lost when the temperature reached 1000
C and was almost lost when the temperature reached 1000  C. The size and spherical morphology of CsPs were almost unchanged after heating, but Cs including RCs, K and Cl were lost, probably diffused away from the CsPs. When the CsPs were heated together with weathered granitic soil that is common in Fukushima, the RCs released from CsPs was sorbed by the surrounding soil. From these results, it is expected that the radioactivity of CsPs will be lost when Rcw including CsPs is burned in incinerators.
C. The size and spherical morphology of CsPs were almost unchanged after heating, but Cs including RCs, K and Cl were lost, probably diffused away from the CsPs. When the CsPs were heated together with weathered granitic soil that is common in Fukushima, the RCs released from CsPs was sorbed by the surrounding soil. From these results, it is expected that the radioactivity of CsPs will be lost when Rcw including CsPs is burned in incinerators.
Yoshigoe, Akitaka; Shiwaku, Hideaki; Kobayashi, Toru; Shimoyama, Iwao; Matsumura, Daiju; Tsuji, Takuya; Nishihata, Yasuo; Kogure, Toshihiro*; Okochi, Takuo*; Yasui, Akira*; et al.
Applied Physics Letters, 112(2), p.021603_1 - 021603_5, 2018/01
Times Cited Count:7 Percentile:29.91(Physics, Applied)A synchrotron radiation photoemission electron microscope (SR-PEEM) was applied to demonstrate pinpoint analysis of micrometer-sized weathered biotite clay particles with artificially adsorbed cesium (Cs) atoms. Despite the insulating properties of the clay, we observed the spatial distributions of constituent elements (Si, Al, Cs, Mg, Fe) without charging issues. We found that Cs atoms were likely to be adsorbed evenly over the entire particle. Spatially-resolved X-ray absorption spectra (XAS) of the Cs M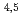 -edge region showed Cs to be present in a monocation state (Cs
-edge region showed Cs to be present in a monocation state (Cs ). Further pinpoint XAS measurements were also performed at the Fe L
). Further pinpoint XAS measurements were also performed at the Fe L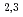 -edge to determine the chemical valence of the Fe atoms. Our results demonstrate the utility of SR-PEEM as a tool for spatially-resolved chemical analyses of various environmental substances, which is not limited by the poor conductivity of samples.
-edge to determine the chemical valence of the Fe atoms. Our results demonstrate the utility of SR-PEEM as a tool for spatially-resolved chemical analyses of various environmental substances, which is not limited by the poor conductivity of samples.
Honda, Mitsunori; Shimoyama, Iwao; Kogure, Toshihiro*; Baba, Yuji; Suzuki, Shinichi; Yaita, Tsuyoshi
ACS Omega (Internet), 2(12), p.8678 - 8681, 2017/12
Times Cited Count:10 Percentile:29.31(Chemistry, Multidisciplinary)Shimoyama, Iwao; Honda, Mitsunori; Kogure, Toshihiro*; Baba, Yuji; Hirao, Norie*; Okamoto, Yoshihiro; Yaita, Tsuyoshi; Suzuki, Shinichi
Photon Factory News, 35(1), p.17 - 22, 2017/05
We introduce Cs-free mineralization (CFM) for Cs removal and reuse of radioactive-contaminated soil in Fukushima and report recent work conducted in the BL27A beamline in Photon Factory. In this work, we investigated compositional and structural changes of Cs-sorbed weathered biotite (WB) before and after heating treatment with addition of NaCl-CaCl salts under low-pressure condition, to study Cs desorption mechanism from clay minerals. X-ray fluorescence spectroscopy clarified that almost all Cs and K were removed with the salts at 700
salts under low-pressure condition, to study Cs desorption mechanism from clay minerals. X-ray fluorescence spectroscopy clarified that almost all Cs and K were removed with the salts at 700  C. On the other hand, Ca increased with heating temperature. X-ray diffraction and transmission electron microscopy analysis clarified that phase transitions from WB to some Ca-rich silicate minerals, e.g., augite, were caused by the heating treatment with the salt. Based on these results, CFM is proposed for Cs removal utilizing the mechanism in which large monovalent cations are discharged with accompanying the phase transition. We also discuss the role of Cl in this reaction showing chemical bonding change of Cl observed using X-ray absorption spectroscopy in the early stage of the chemical reaction.
C. On the other hand, Ca increased with heating temperature. X-ray diffraction and transmission electron microscopy analysis clarified that phase transitions from WB to some Ca-rich silicate minerals, e.g., augite, were caused by the heating treatment with the salt. Based on these results, CFM is proposed for Cs removal utilizing the mechanism in which large monovalent cations are discharged with accompanying the phase transition. We also discuss the role of Cl in this reaction showing chemical bonding change of Cl observed using X-ray absorption spectroscopy in the early stage of the chemical reaction.
 -XRD with synchrotron radiation
-XRD with synchrotron radiationMotai, Satoko*; Mukai, Hiroki*; Watanuki, Tetsu; Owada, Kenji; Fukuda, Tatsuo; Machida, Akihiko; Kuramata, Chisaki*; Kikuchi, Ryosuke*; Yaita, Tsuyoshi; Kogure, Toshihiro*
Journal of Mineralogical and Petrological Sciences, 111(5), p.305 - 312, 2016/10
Times Cited Count:17 Percentile:48.01(Mineralogy)no abstracts in English
Kogure, Toshihiro*; Yamaguchi, Noriko*; Segawa, Hiroyo*; Mukai, Hiroki*; Motai, Satoko*; Akiyama, Kotone*; Mitome, Masanori*; Hara, Toru*; Yaita, Tsuyoshi
Microscopy, 65(5), p.451 - 459, 2016/10
Times Cited Count:57 Percentile:96.45(Microscopy)Mukai, Hiroki*; Hirose, Atsushi*; Motai, Satoko*; Kikuchi, Ryosuke*; Tanoi, Keitaro*; Nakanishi, Tomoko*; Yaita, Tsuyoshi; Kogure, Toshihiro*
Scientific Reports (Internet), 6, p.21543_1 - 21543_7, 2016/02
Times Cited Count:138 Percentile:96.12(Multidisciplinary Sciences)Mukai, Hiroki*; Hatta, Tamao*; Kitazawa, Hideaki*; Yamada, Hirohisa*; Yaita, Tsuyoshi; Kogure, Toshihiro*
Environmental Science & Technology, 48(22), p.13053 - 13059, 2014/12
Times Cited Count:117 Percentile:93.89(Engineering, Environmental)no abstracts in English
Murakami, Takashi*; Kogure, Toshihiro*; ; Onuki, Toshihiko
American Mineralogist, 83, p.1209 - 1219, 1998/00
no abstracts in English
; Murakami, Takashi*; Kogure, Toshihiro*; Isobe, Hiroshi; Sato, Tsutomu
Mat. Res. Soc. Symp. Proc., 506, p.839 - 846, 1998/00
no abstracts in English
Shimoyama, Iwao; Honda, Mitsunori; Kogure, Toshihiro*; Baba, Yuji; Hirao, Norie*; Okamoto, Yoshihiro; Yaita, Tsuyoshi; Suzuki, Shinichi
no journal, ,
The decontamination method has not been established for soil contaminated with radioactive Cs. We adopted weathered biotite (WB) which sorbed non-radioactive  Cs as a model contaminated soil and applied heating treatment to the WB at 700
Cs as a model contaminated soil and applied heating treatment to the WB at 700 C under a low pressure condition with CaCl
C under a low pressure condition with CaCl or KCl salt. X-ray fluorescence analysis showed that almost all Cs was removed after the heating at 700
or KCl salt. X-ray fluorescence analysis showed that almost all Cs was removed after the heating at 700 C with CaCl
C with CaCl and Ca became dominant with temperature. X-ray diffraction and transmission electron microscopy analysis indicated some phase transition from WB after the heating treatment. These results suggest a model that Ca derived from the salt induced the formation of augite, and that Cs
and Ca became dominant with temperature. X-ray diffraction and transmission electron microscopy analysis indicated some phase transition from WB after the heating treatment. These results suggest a model that Ca derived from the salt induced the formation of augite, and that Cs were eliminated from the product as these large-size cations cannot constitute augite. Based on this model, we propose Cs free mineralization which can achieve decontamination and reuse of soil in Fukushima.
were eliminated from the product as these large-size cations cannot constitute augite. Based on this model, we propose Cs free mineralization which can achieve decontamination and reuse of soil in Fukushima.
 mixed salt
mixed saltShimoyama, Iwao; Honda, Mitsunori; Kogure, Toshihiro*; Okamoto, Yoshihiro; Yaita, Tsuyoshi; Suzuki, Shinichi
no journal, ,
X-ray fluorescence (XRF) and X-ray diffraction (XRD) were employed to study bulk composition and structure changes of weathered biotite (WB) before and after low-pressure sublimation processing with NaCl-CaCl mixed salt. XRF showed that almost all Cs was desorbed from Cs-saturated WB after heating at 700
mixed salt. XRF showed that almost all Cs was desorbed from Cs-saturated WB after heating at 700  C under a low-pressure (14 Pa) and XRD showed that WB disappeared and augite appeared as the dominant product. We propose a new idea, Cs-free mineralization method, for reduction of Cs-contaminated soil in Fukushima based on these results.
C under a low-pressure (14 Pa) and XRD showed that WB disappeared and augite appeared as the dominant product. We propose a new idea, Cs-free mineralization method, for reduction of Cs-contaminated soil in Fukushima based on these results.
 and KCl additives on soil decontamination by cesium-free mineralization
and KCl additives on soil decontamination by cesium-free mineralizationShimoyama, Iwao; Honda, Mitsunori; Kogure, Toshihiro*; Baba, Yuji; Yaita, Tsuyoshi; Okamoto, Yoshihiro
no journal, ,
We examined decontamination of radioactively contaminated soil in Fukushima using cesium free mineralization (CFM) with CaCl or KCl additives. We analyzed radioactive concentration and structural changes of the samples using a NaI detector and an X-ray diffractometer. In the case of CaCl
or KCl additives. We analyzed radioactive concentration and structural changes of the samples using a NaI detector and an X-ray diffractometer. In the case of CaCl , decontamination ratio R
, decontamination ratio R showed similar tendency for both air and low-pressure heating, and R
showed similar tendency for both air and low-pressure heating, and R reached to 97% after heating at 790
reached to 97% after heating at 790 C. X-ray diffraction (XRD) showed that transformation from clay minerals to other silicate minerals occurred by the heat treatments and products depend on the pressure condition during the heating. In the case of KCl, R
C. X-ray diffraction (XRD) showed that transformation from clay minerals to other silicate minerals occurred by the heat treatments and products depend on the pressure condition during the heating. In the case of KCl, R was higher for low-pressure heating than for air heating and reached to 83% at 790
was higher for low-pressure heating than for air heating and reached to 83% at 790 C. XRD showed that clay minerals remained after the heating treatment. These results indicate that CFM is effective for decontamination of actual Fukushima soil and different mechanisms of Cs removal from contaminated soil exist for heating treatments with KCl and CaCl
C. XRD showed that clay minerals remained after the heating treatment. These results indicate that CFM is effective for decontamination of actual Fukushima soil and different mechanisms of Cs removal from contaminated soil exist for heating treatments with KCl and CaCl .
.
Shimoyama, Iwao; Honda, Mitsunori; Kogure, Toshihiro*; Hirao, Norie*; Baba, Yuji; Okamoto, Yoshihiro; Yaita, Tsuyoshi; Suzuki, Shinichi
no journal, ,
We adopted low pressure heating treatments for Cs saturated and sorbed weathered biotite (WB) with some alkaline salts, e.g., CaCl , KCl, NaCl to develop decontamination method for radioactive Cs contaminated soil in Fukushima, and analyzed the samples before and after the treatments using X-ray fluorescence analysis, X-ray diffraction, transmission electron microscopy, and thermal desorption spectroscopy. When we heated WB at 700
, KCl, NaCl to develop decontamination method for radioactive Cs contaminated soil in Fukushima, and analyzed the samples before and after the treatments using X-ray fluorescence analysis, X-ray diffraction, transmission electron microscopy, and thermal desorption spectroscopy. When we heated WB at 700  C with CaCl
C with CaCl , the structure of WB completely decomposed, and almost 100% of Cs and K sorbed in WB were removed from the sample. We observed that the Ca content in the product increased with temperature, which induced phase change from WB to augite and wadalite. These results indicate that Cs
, the structure of WB completely decomposed, and almost 100% of Cs and K sorbed in WB were removed from the sample. We observed that the Ca content in the product increased with temperature, which induced phase change from WB to augite and wadalite. These results indicate that Cs and K
and K that are large monovalent cations were discharged by formation of these products. Meanwhile, the addition of KCl removed only 55% of Cs at 700
that are large monovalent cations were discharged by formation of these products. Meanwhile, the addition of KCl removed only 55% of Cs at 700  C through ion exchange reaction keeping the layered structure of WB. Based on these phenomena, we propose the idea of Cs free mineralization that enables us to decontaminate soil using phase change from clay minerals to other minerals by reactions with alkaline salts.
C through ion exchange reaction keeping the layered structure of WB. Based on these phenomena, we propose the idea of Cs free mineralization that enables us to decontaminate soil using phase change from clay minerals to other minerals by reactions with alkaline salts.
Yamamoto, Yuhei; Aosai, Daisuke; Mizuno, Takashi; Watanabe, Katsuaki*; Kogure, Toshihiro*; Suzuki, Yohei*
no journal, ,
no abstracts in English