Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tobitsuka, Sachiko; Iijima, Kazuki; Kohara, Yukitoshi*
Proceedings of 29th International Symposium of Scientific Basis for Nuclear Waste Management (MRS 2005), 0 Pages, 2005/09
The influence of humic acid for the solubility of tetravalent neptunium was investigated under reducing condition, by comparing at various pH. All of experiments were carried in an argon glove-box except analyses. At pH5, 8 and 9, at 20-24 degree C, Np solution in 1E-5 or 1E-3 mol/l was mixed with 0, 5-1,000 mg/l of purified Aldrich humic acid (PAHA). The ionic strength was 0.25 which was brought by 0.1 mol/l NaClO and 0.05 mol/l Na
and 0.05 mol/l Na S
S O
O added as a reductant to keep Np in tetravalent. The concentration of Np was quantified periodically by alpha spectrometry or ICP-MS. With the acid-base titration, analyzed by NICA-Donnan model which assumes the carboxyl group and the phenolic ydroxyl group as two main functional groups, the charge density of PAHA was 2.84, 4.36, 4.78 meq/g at pH5, 8, 9, respectively. In all cases, the total Np concentration ([Np]t) increases with increasing PAHA concentration. In the case of 1E-5 mol/l of initial Np concentration ([Np]
added as a reductant to keep Np in tetravalent. The concentration of Np was quantified periodically by alpha spectrometry or ICP-MS. With the acid-base titration, analyzed by NICA-Donnan model which assumes the carboxyl group and the phenolic ydroxyl group as two main functional groups, the charge density of PAHA was 2.84, 4.36, 4.78 meq/g at pH5, 8, 9, respectively. In all cases, the total Np concentration ([Np]t) increases with increasing PAHA concentration. In the case of 1E-5 mol/l of initial Np concentration ([Np]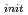 especially, [Np]t increases almost up to [Np]
especially, [Np]t increases almost up to [Np]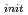 in 50 mg/l PAHA at pH8, 9, or 25 mg/l at pH5. Even in the case of PAHA 5 mg/l, [Np]t becomes one order or more higher than that in the absence of PAHA. The apparent stability constant
in 50 mg/l PAHA at pH8, 9, or 25 mg/l at pH5. Even in the case of PAHA 5 mg/l, [Np]t becomes one order or more higher than that in the absence of PAHA. The apparent stability constant
Fujiwara, Kenso; Kohara, Yukitoshi*; Mori, Takashi
JNC TN8400 2004-021, 32 Pages, 2004/12
In support of the performance assessment of geological disposal of high-level radioactive waste, solubility measurement of tetravalent neptunium (Np(IV)) hydroxide in reducing condition was performed. Np stock solution was reduced by using H gas and platinum black (catalyst). Solubility test were perfomed by oversaturation method. Sample solution of 1.0 M NaClO
gas and platinum black (catalyst). Solubility test were perfomed by oversaturation method. Sample solution of 1.0 M NaClO was spiked by the Np(IV) solution. The pHc value was adjusted around 3 with NaOH and HClO
was spiked by the Np(IV) solution. The pHc value was adjusted around 3 with NaOH and HClO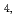 and the sealed sample solution was standing at room tempreture (23
and the sealed sample solution was standing at room tempreture (23  2
2 C) in the shaded box avoiding photo-oxidation. The concentration of Np(IV) was measured by alpha-spectrometry. The solubility decreased with increasing pHc. It can be seen that the hydrolysis species are predominantly formed with the slope of solubility. It can be seen that the hydrolysis species are predominantly formed with the slope of solubility. From the obtained results, the solubility product (K
C) in the shaded box avoiding photo-oxidation. The concentration of Np(IV) was measured by alpha-spectrometry. The solubility decreased with increasing pHc. It can be seen that the hydrolysis species are predominantly formed with the slope of solubility. It can be seen that the hydrolysis species are predominantly formed with the slope of solubility. From the obtained results, the solubility product (K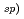 of Np hydroxide at I = 1.0 were determined. The experimental values were extrapolated to the standard state (I = 0) by using the specific interaction theory (SIT), and the solubility product of Np hydroxide was determined to be logKsp° = -56.2
of Np hydroxide at I = 1.0 were determined. The experimental values were extrapolated to the standard state (I = 0) by using the specific interaction theory (SIT), and the solubility product of Np hydroxide was determined to be logKsp° = -56.2  0.3.
0.3.
Iijima, Kazuki; Tobitsuka, Sachiko; Kohara, Yukitoshi*
JNC TN8400 2004-005, 46 Pages, 2004/04
Complexation behavior between humic acid (HA), which is one of the natural organic matter in gourndwater, and tetravalent neptunium (Np(IV)) was investigated by batch type copmlexation experiments and solvent extraction by TTA xylene, focused on the effect concentration and molecular weight of HA. In addition, proton dissociation behavior from HA was evaluated by acid-base titration and its results was used for the calculation of stability constant of Np(IV) and HA.
Fujiwara, Kenso; Kohara, Yukitoshi*
Advanced Science Research(ARS2004) Advances in Heavy Elements Microbiology Research p.37, P. 37, 2004/00
Focusing on the cover layer materials (as the Radon Barrier Materials), which could have the effect to restrain the radon from scattering into the air and the effect of the radiation shielding, we produced the radon barrier materials with crude bentonite on an experimental basis, using the rotary type comprehensive unit for grinding and mixing, through which we carried out the evaluation of the characteristics thereof.
Kitamura, Akira; Kohara, Yukitoshi*
Radiochimica Acta, 92(9-11), p.583 - 588, 2004/00
Times Cited Count:12 Percentile:61.53(Chemistry, Inorganic & Nuclear)The solubility of Np(IV) in carbonate media was studied for determination of thermodynamic data. It was found that the solubility of Np(IV) increased with increasing CT.
Tobitsuka, Sachiko; Kohara, Yukitoshi*; Iijima, Kazuki; Sato, Haruo
JNC TN8400 2003-018, 53 Pages, 2003/06
Humic substances which are humic acid, fulvic acid and humin, exist in the groundwater and the pore water inside of compacted bentonite as the natural organic matters. These are considered that these make increase the solubility of radionuclieds, or decre
Kitamura, Akira; Kohara, Yukitoshi*
Dai-9-Kai Chishitsu Kankyo Niokeru Akuchinido Oyobi Kaku Bunretsu Seiseibutsu No Kagaku Oyobi Iko Kyodo Ni Kansuru Kokusai Gakkai, 0 Pages, 2003/00
None
Kitamura, Akira; Kohara, Yukitoshi*
JNC TN8400 2001-006, 35 Pages, 2001/01
Solubility of Np(IV) in carnonatemedia under reducing conditions was studied. The concentration of dissolved Np(Ⅳ) was measured with ionic strengths of 0.5M(M  mol
mol m
m ) and 1.0 M, hydrogen-ion concentration exponent (pH
) and 1.0 M, hydrogen-ion concentration exponent (pH = -log[H
= -log[H ) from 8.5 to 13 and with the total carbonate concentration (C
) from 8.5 to 13 and with the total carbonate concentration (C ) from 0.005 M to 0.1 M by the oversaturation method. The reducing agent used was sodium dithionite. It was found that the solubility of Np(IV) decreased with increasing pH
) from 0.005 M to 0.1 M by the oversaturation method. The reducing agent used was sodium dithionite. It was found that the solubility of Np(IV) decreased with increasing pH and increased with increasing C
and increased with increasing C and the ionie strength. On the basis of analysis of the solubility data, the dominant aqueous species of Np(IV) were expected to be Np(CO
and the ionie strength. On the basis of analysis of the solubility data, the dominant aqueous species of Np(IV) were expected to be Np(CO )
) (OH)
(OH)
 and Np(CO
and Np(CO )
) (OH)
(OH)
 in the present study. The apparent equilibrium constants of the carbonatohydroxo complexes were obtained in the respective ionic strength of 0.5 M and 1.0 M, and the thermodynamic equmbrium constants at the zero ionic strength, were extrapolated by a model called "specific ion interaction theory (SIT)". Comparing with the literature data of thermodynamic equilibrium constants, the reasonableness of the present data was discussed.
in the present study. The apparent equilibrium constants of the carbonatohydroxo complexes were obtained in the respective ionic strength of 0.5 M and 1.0 M, and the thermodynamic equmbrium constants at the zero ionic strength, were extrapolated by a model called "specific ion interaction theory (SIT)". Comparing with the literature data of thermodynamic equilibrium constants, the reasonableness of the present data was discussed.
; Kohara, Yukitoshi*; *;
JNC TN8400 99-089, 21 Pages, 1999/11
Understanding the migration behavior of radionuclides in compacted bentonites is important for the performance assessment of geological disposal of high-level radioactive waste. In the Second Progress Report, the distribution coefficients of radionuclides on bentonites have been determined based on apparent diffusion coefficients(D ) obtained for compacted bentonites. Therefore, the apparent diffusion coefficient is one of the most important parameters for the performance assessment. In this report, the diffusion behavior of plutonium and americium in compacted bentonites was studied. The diffusion experiments of Pu and Am in compacted bentonite (Kunigel V1) were conducted by in-diffusion method under aerobic conditions at room temperature. The following results were acquired. (1)The apparent diffusion coefficients of Pu obtained for compacted bentonite with a dry density of 0.4 Mg/m
) obtained for compacted bentonites. Therefore, the apparent diffusion coefficient is one of the most important parameters for the performance assessment. In this report, the diffusion behavior of plutonium and americium in compacted bentonites was studied. The diffusion experiments of Pu and Am in compacted bentonite (Kunigel V1) were conducted by in-diffusion method under aerobic conditions at room temperature. The following results were acquired. (1)The apparent diffusion coefficients of Pu obtained for compacted bentonite with a dry density of 0.4 Mg/m were in the range of 2.0
were in the range of 2.0  10
10 to 2.2
to 2.2  10
10 m
m /s. These values were similar to those for compacted smectite (Kunipia F) reported by JNC in the past. (2)The apparent diffusion coefficients of Am obtained for compacted bentonite with a dry density of 2.0 Mg/m
/s. These values were similar to those for compacted smectite (Kunipia F) reported by JNC in the past. (2)The apparent diffusion coefficients of Am obtained for compacted bentonite with a dry density of 2.0 Mg/m were in the range of 2.7
were in the range of 2.7  10
10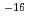 to 1.2
to 1.2  10
10 m
m /s. These values were consistent with the dependency of D
/s. These values were consistent with the dependency of D on density reported by JNC in the past. It is difficult to obtain accurate diffusion coefficient of Pu and Am due to quite slow diffusivity. The apparent diffusion coefficients obtained for individual, conditions in this study include uncertainty to some extent (one order of magnitude). The concentration profile of Pu was confirmed to have complicated shape composed of three parts, which implied complexity of diffusion behavior. To understand diffusion mechanisms of these elements in compacted bentonites, more accurate diffusion data and information focused on relation between species and diffusion behavior are essential.
on density reported by JNC in the past. It is difficult to obtain accurate diffusion coefficient of Pu and Am due to quite slow diffusivity. The apparent diffusion coefficients obtained for individual, conditions in this study include uncertainty to some extent (one order of magnitude). The concentration profile of Pu was confirmed to have complicated shape composed of three parts, which implied complexity of diffusion behavior. To understand diffusion mechanisms of these elements in compacted bentonites, more accurate diffusion data and information focused on relation between species and diffusion behavior are essential.
Shibutani, Tomoki; Kohara, Yukitoshi*; Oda, Chie; Kubota, Mitsuru*; ; Shibata, Masahiro
JNC TN8400 99-066, 75 Pages, 1999/11
The physico-chemical characteristics of purified Na-smectite and protonation/deprotonation behavior of smectite surface in different concentrations of NaCl solutions were studied to identify the reaction mechanism of smectite-water interaction for performance assessment of HLW geological disposal system. The Na-smectite was purified from Kunipia F (obtained from Kunimine Industries Co. Ltd. Japan). In this smectite, small amount of quartz was detected as an impurity by X-ray diffraction. As a result of XRD and chemical analysis of the smectite, it was found that exchangeable sites in smectite inter-layer were occupied by Na+. Cation exchange capacity (CEC) was measured as 1.108meq g by using ammonium acetate. The N
by using ammonium acetate. The N -BET surface area of smectite was 50
-BET surface area of smectite was 50 58m
58m g
g . Protonation/deprotonation behavior of smectite was studied for 0.01, 0.1 and 0.5M NaCl by using titration and back titration method. Total amount of H
. Protonation/deprotonation behavior of smectite was studied for 0.01, 0.1 and 0.5M NaCl by using titration and back titration method. Total amount of H consumption increased with decreasing pH for all NaCl concentrations, and the titration curves in these solutions showed similar trend in the pH range of 6-11. On the other hand, total amount of H
consumption increased with decreasing pH for all NaCl concentrations, and the titration curves in these solutions showed similar trend in the pH range of 6-11. On the other hand, total amount of H consumption increased with decreasing NaCl concentration in the pH range of 2-6. The dominant sorption mechanism of H
consumption increased with decreasing NaCl concentration in the pH range of 2-6. The dominant sorption mechanism of H on smectite was different between pH
on smectite was different between pH 6 and pH
6 and pH 6, and it can be considered that H
6, and it can be considered that H was sorbed on the same site as Na
was sorbed on the same site as Na for pH
for pH 6 and different site from Na
6 and different site from Na for pH
for pH 6. The prediction of protonation/deprotonation behavior of smectite for 0.01, 0.1 and 0.5M NaCl was carried out based on ion exchange and surface complexation models. The sorption sites were assumed as inter-layer and crystal edge site. The site concentrations for ion exchange and surface complexation were calculated from CEC. The reaction constants were consequently calculated by fitting of experimental results as follows. ...
6. The prediction of protonation/deprotonation behavior of smectite for 0.01, 0.1 and 0.5M NaCl was carried out based on ion exchange and surface complexation models. The sorption sites were assumed as inter-layer and crystal edge site. The site concentrations for ion exchange and surface complexation were calculated from CEC. The reaction constants were consequently calculated by fitting of experimental results as follows. ...
Ashida, Takashi; Kohara, Yukitoshi*; Shibutani, Tomoki; Yui, Mikazu
PNC TN8410 98-014, 30 Pages, 1998/03
None
; ; Kohara, Yukitoshi*; Uchidate, Nobuyuki*; Yui, Mikazu; Ishikawa, Hirohisa
PNC TN8410 98-010, 68 Pages, 1997/12
None
Sato, Haruo; Ashida, Takashi; Kohara, Yukitoshi*; Yui, Mikazu; Umeki, Hiroyuki; Ishiguro, Katsuhiko
PNC TN8410 92-164, 31 Pages, 1992/09
None
Sato, Haruo; Ashida, Takashi; Yui, Mikazu; Kohara, Yukitoshi*
16th Scientific Basis Symposium for Radioactive Waste Management, 0 Pages, 1992/00
None
Sasaki, Noriaki; Ishikawa, Hirohisa; Idemitsu, Kazuya; Arai, Takashi; Hirose, Ikuro; Miyahara, Kaname; Ashida, Takashi; Oi, Takao; Kohara, Yukitoshi*
PNC TN8420 88-005, 96 Pages, 1988/07
None
Fujiwara, Kenso; Kohara, Yukitoshi*
no journal, ,
no abstracts in English
Fujiwara, Kenso; Kohara, Yukitoshi*
no journal, ,
no abstracts in English
Fujiwara, Kenso; Odakura, Makoto; Kuroha, Mitsuhiko; Kohara, Yukitoshi*; Kikuchi, Hiroshi*
no journal, ,
The purpose of this study is to alteration-phase formation and associated elemental release furing aqueous corrosion of high-level waste(HLW) glass. Static corrosion tests were formed at high temperature (90, 120 degrees) alkali (pH 11, 12, 13) condition. Crystalline alteration-phase formed in the corroded glass were made. Most of Cs in the glass is retained in the alteration-phases into amorphous phases and pollucite.
Fujiwara, Kenso; Kohara, Yukitoshi*; Okazaki, Mitsuhiro*; Suzuki, Yasuyuki*
no journal, ,
The stability constants of Np(IV) with Ca and Si are measured by solubility method. In the case of coexistence of Ca or Si under high pH region, the solubility of tetravalent actinide was higher than the case of without Ca or Si in a recent study. However, in the case of Np(IV), the experiment was not measured. Therefore, the solubility of Np(IV) was measured with Ca or Si in the high pH region, the stability constants of (Ca Np(OH)
Np(OH)
 ) and Si of Ca and the Np and complex (Np(OH)nSi
) and Si of Ca and the Np and complex (Np(OH)nSi O
O (OH)
(OH) etc) was calculated by solubility of Np(IV).
etc) was calculated by solubility of Np(IV).
Fujiwara, Kenso; Iijima, Kazuki; Mitsui, Seiichiro; Odakura, Makoto; Kohara, Yukitoshi*; Kikuchi, Hiroshi*
no journal, ,
The in-diffusion experiment was carried out for about 15 years to evaluate the migration behavior of multivalent actinide and lanthanide elements by using fully radioactive waste glass and bentonite. The experimental concentrations of Am, Cm and Pu in contact with compacted sodium bentonite were good agreement with the solubilities calculated by thermodynamic data. The profiles of Am and Cm show two different slopes which be fitted by simple one-dimensional diffusion model each other.