Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hidaka, Koshi*; Kimura, Toru*; Abdel-Rahman, H. M.*; Nguyen, J.-T.*; McDaniel, K. F.*; Kohlbrenner, W. E.*; Molla, A.*; Adachi, Motoyasu; Tamada, Taro; Kuroki, Ryota; et al.
Journal of Medicinal Chemistry, 52(23), p.7604 - 7617, 2009/07
Times Cited Count:19 Percentile:44.7(Chemistry, Medicinal)A series of HIV protease inhibitor based on the allophenylnorstatine structure with various P2' moieties were synthesized. Among these analogues, we discovered that a small allyl group would maintain potent enzyme inhibitory activity compared to that of the 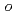 -methylbenzyl moiety in clinical candidate 1 (KNI-764, also known as JE-2147, AG-1776 or SM-319777). Introduction of an anilinic amino group to 2 (KNI-727) improved water-solubility and anti-HIV-1 activity. X-ray crystallographic analysis of 13k (KNI-1689) with a
-methylbenzyl moiety in clinical candidate 1 (KNI-764, also known as JE-2147, AG-1776 or SM-319777). Introduction of an anilinic amino group to 2 (KNI-727) improved water-solubility and anti-HIV-1 activity. X-ray crystallographic analysis of 13k (KNI-1689) with a  -methallyl group at P2' position revealed hydrophobic interactions with Ala28, Ile84, and Ile50' similar to that of 1. The presence of an additional methyl group on the allyl group in compound 13k significantly increased anti-HIV activity over 1, while providing a rational drug design for structural minimization and improving membrane permeability.
-methallyl group at P2' position revealed hydrophobic interactions with Ala28, Ile84, and Ile50' similar to that of 1. The presence of an additional methyl group on the allyl group in compound 13k significantly increased anti-HIV activity over 1, while providing a rational drug design for structural minimization and improving membrane permeability.