Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 and Ag
and Ag clusters
clustersKoizumi, Akihito*; Tachikawa, Masanori*; Shiga, Motoyuki
Chemical Physics, 419, p.44 - 49, 2013/06
Times Cited Count:1 Percentile:2.56(Chemistry, Physical)The structures and infrared spectra of Ag (H
(H O)
O)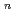 (
( = 1-4) and Cu
= 1-4) and Cu (H
(H O) are studied by
O) are studied by 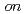 -
-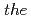 -
-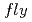
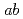
 MD,
MD, 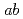
 PIMD, and
PIMD, and 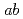
 RPMD simulations. It is found that the anharmonicity due to nuclear quantum and thermal effects acts differently depending on the system as well as the type of vibrational mode. In the low-frequency region, the spectra become a broad band as the cluster size increases due to the complex mode couplings, which is qualitatively different from the harmonic spectra. In contrast, the HOH bending modes are systematically red-shifted by a small amount due to the anharmonicity. The OH stretching modes are found to be also red-shifted, but the amount of shift is more dependent on the system. Consequently, these effects cannot be described by unique scaling of harmonic frequencies.
RPMD simulations. It is found that the anharmonicity due to nuclear quantum and thermal effects acts differently depending on the system as well as the type of vibrational mode. In the low-frequency region, the spectra become a broad band as the cluster size increases due to the complex mode couplings, which is qualitatively different from the harmonic spectra. In contrast, the HOH bending modes are systematically red-shifted by a small amount due to the anharmonicity. The OH stretching modes are found to be also red-shifted, but the amount of shift is more dependent on the system. Consequently, these effects cannot be described by unique scaling of harmonic frequencies.
 O)
O)Koizumi, Akihito*; Suzuki, Kimichi*; Shiga, Motoyuki; Tachikawa, Masanori*
International Journal of Quantum Chemistry, 112(1), p.136 - 139, 2012/01
Times Cited Count:0 Percentile:0.05(Chemistry, Physical)Ab initio path integral molecular dynamics simulation of MOH(H O) (M= Cu, Ag, and Au) clusters has been carried out to analyze how the hydrogen-bonded proton can be affected by the counter noble metal cation. The CuOH(H
O) (M= Cu, Ag, and Au) clusters has been carried out to analyze how the hydrogen-bonded proton can be affected by the counter noble metal cation. The CuOH(H O) cluster forms no hydrogen bonded structure even for the static equilibrium structures. Contrary to our previous paper of hydrated alkali metal hydroxide clusters, proton transferred distribution was not observed because of the high potential barrier heights of MOH(H
O) cluster forms no hydrogen bonded structure even for the static equilibrium structures. Contrary to our previous paper of hydrated alkali metal hydroxide clusters, proton transferred distribution was not observed because of the high potential barrier heights of MOH(H O).
O).
Daido, Masashi*; Koizumi, Akihito*; Shiga, Motoyuki; Tachikawa, Masanori*
Theoretical Chemistry Accounts, 130(2-3), p.385 - 391, 2011/10
Times Cited Count:6 Percentile:13.82(Chemistry, Physical)The structure of Watson-Crick type guanine-cytosine (G-C) base pair has been studied by classical hybrid Monte Carlo (HMC) and quantum path integral hybrid Monte Carlo (PIHMC) simulations on the semiempirical potential energy surface. For the three NH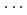 X hydrogen-bonded moieties, the intramolecular NH bonds are found systematically longer while the H
X hydrogen-bonded moieties, the intramolecular NH bonds are found systematically longer while the H X distance shorter in the PIHMC simulation than in the HMC simulation. We found that the hydrogen bonded length N
X distance shorter in the PIHMC simulation than in the HMC simulation. We found that the hydrogen bonded length N X correlates with the H
X correlates with the H X distance, but not with the NH distance. A correlation is also between the neighboring hydrogen bonds in the G-C base pair.
X distance, but not with the NH distance. A correlation is also between the neighboring hydrogen bonds in the G-C base pair.
Koizumi, Akihito*; Suzuki, Kimichi*; Shiga, Motoyuki; Tachikawa, Masanori*
Journal of Chemical Physics, 134(3), p.031101_1 - 031101_3, 2011/01
Times Cited Count:14 Percentile:42.14(Chemistry, Physical)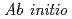 path integral molecular dynamics simulation of M
path integral molecular dynamics simulation of M H
H O
O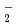 (M = Li, Na, and K) has been carried out to study how the structure and dynamics of low-barrier hydrogen-bonded Zundel anion, H
(M = Li, Na, and K) has been carried out to study how the structure and dynamics of low-barrier hydrogen-bonded Zundel anion, H O
O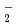 , can be affected by the counter alkali metal cation, M
, can be affected by the counter alkali metal cation, M . Our simulation predicts that the quantum proton transfer in Zundel anion can be strongly coupled to the motion of counter cation located nearby. A smaller cation can induce larger structural distortion of the Zundel anion fragment making the proton transfer barrier higher, and hence lowers the vibration excitation energy. It is also argued that a large H/D isotope effect is present.
. Our simulation predicts that the quantum proton transfer in Zundel anion can be strongly coupled to the motion of counter cation located nearby. A smaller cation can induce larger structural distortion of the Zundel anion fragment making the proton transfer barrier higher, and hence lowers the vibration excitation energy. It is also argued that a large H/D isotope effect is present.