Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...

Machida, Akihiko; Honda, Mitsunori*; Hattori, Takanori; Sano, Asami; Watanuki, Tetsu; Katayama, Yoshinori; Aoki, Katsutoshi; Komatsu, Kazuki*; Arima, Hiroshi*; Oshita, Hidetoshi*; et al.
Physical Review Letters, 108(20), p.205501_1 - 205501_5, 2012/05
Times Cited Count:17 Percentile:67.41(Physics, Multidisciplinary)Hydrogen atoms absorbed in a metal occupy the interstitial sites of the metal lattice. In an fcc metal lattice, each metal atom has two tetrahedral (T) and one octahedral (O) sites that can accommodate hydrogen. Rare-earth metal La forms T-site occupied LaH and fully occupied LaH
and fully occupied LaH . O-site occupied or NaCl-type monohydride has yet to be reported for rare-earth metals. Previous X-ray diffraction measurements revealed the pressure-induced decomposition of an fcc-LaH
. O-site occupied or NaCl-type monohydride has yet to be reported for rare-earth metals. Previous X-ray diffraction measurements revealed the pressure-induced decomposition of an fcc-LaH into H-rich and H-poor phases around 11 GPa. The present neutron diffraction measurements on LaD
into H-rich and H-poor phases around 11 GPa. The present neutron diffraction measurements on LaD confirm the formation of NaCl-type LaD as a counterpart of the D-rich LaD
confirm the formation of NaCl-type LaD as a counterpart of the D-rich LaD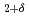 by disproportionation. First-principle calculations demonstrate that the NaCl-type LaH is stabilized at high pressures. Finding the NaCl-type LaH will pave the way for investigations on the site-dependent nature of hydrogen-metal interactions.
by disproportionation. First-principle calculations demonstrate that the NaCl-type LaH is stabilized at high pressures. Finding the NaCl-type LaH will pave the way for investigations on the site-dependent nature of hydrogen-metal interactions.
Kamakura, Nozomu; Takeda, Yukiharu; Saito, Yuji; Yamagami, Hiroshi; Tsubota, Masami*; Paik, B.*; Ichikawa, Takayuki*; Kojima, Yoshitsugu*; Muro, Takayuki*; Kato, Yukako*; et al.
Physical Review B, 83(3), p.033103_1 - 033103_4, 2011/01
Times Cited Count:5 Percentile:25.35(Materials Science, Multidisciplinary)The electronic structure of lithium amide, which is lightweight complex hydride expected as a high-capacity hydrogen storage material, is investigated by N 1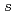 soft X-ray emission spectroscopy (XES) and absorption spectroscopy (XAS). The overall feature of the electronic structure of lithium amide by the XES and XAS is consistent with the band calculation, while the strongly hybridized state with H 1
soft X-ray emission spectroscopy (XES) and absorption spectroscopy (XAS). The overall feature of the electronic structure of lithium amide by the XES and XAS is consistent with the band calculation, while the strongly hybridized state with H 1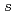 is located at higher binding energy than the band calculation.
is located at higher binding energy than the band calculation.
 -edges
-edgesIshimatsu, Naoki*; Sasada, Ryohei*; Maruyama, Hiroshi*; Ichikawa, Takayuki*; Miyaoka, Hiroki*; Kimura, Toru*; Tsubota, Masami*; Kojima, Yoshitsugu*; Tsumuraya, Takao*; Oguchi, Tamio*; et al.
Journal of Physics; Conference Series, 190, p.012070_1 - 012070_4, 2009/11
Times Cited Count:5 Percentile:79.33(Physics, Condensed Matter)We have investigated the effect of hydrogenation on La 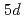 and
and 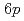 electronic states in metallic LaH
electronic states in metallic LaH by X-ray absorption near edge structure at the La
by X-ray absorption near edge structure at the La  -edges. As the hydrogen content
-edges. As the hydrogen content  increases from 0 to 2.6, white-line intensity at the La
increases from 0 to 2.6, white-line intensity at the La 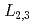 -edges shows a remarkable increase in the range of
-edges shows a remarkable increase in the range of 
 2.0. This is interpreted as the increase in La
2.0. This is interpreted as the increase in La 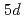 hole induced by interstitial H atoms on the octahedral sites. On the other hand, the shoulder structure at the La
hole induced by interstitial H atoms on the octahedral sites. On the other hand, the shoulder structure at the La 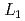 -edge disappears in the process of
-edge disappears in the process of  = 0.0
= 0.0  2.0, indicating that the
2.0, indicating that the  -
-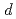 hybridization is weakened by H atoms on the tetrahedral sites. This study demonstrates that H atoms on the two interstitial H sites provide different contribution to the modification of the electronic states.
hybridization is weakened by H atoms on the tetrahedral sites. This study demonstrates that H atoms on the two interstitial H sites provide different contribution to the modification of the electronic states.
Kamakura, Nozomu; Takeda, Yukiharu; Saito, Yuji; Yamagami, Hiroshi; Tsubota, Masami*; Paik, B.*; Ichikawa, Takayuki*; Kojima, Yoshitsugu*; Muro, Takayuki*; Kinoshita, Toyohiko*
no journal, ,
Lithium amide (LiNH ) is lightweight complex hydride expected as a high-capacity hydrogen storage material. The electronic structure of lithium amide (LiNH
) is lightweight complex hydride expected as a high-capacity hydrogen storage material. The electronic structure of lithium amide (LiNH ) is investigated by soft X-ray emission spectroscopy (XES) and absorption spectroscopy (XAS). The unoccupied and occupied parts of the N 2
) is investigated by soft X-ray emission spectroscopy (XES) and absorption spectroscopy (XAS). The unoccupied and occupied parts of the N 2 partial density of states are studied by N 1
partial density of states are studied by N 1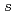 XAS in the fluorescence yield mode and XES using soft X-ray (h
XAS in the fluorescence yield mode and XES using soft X-ray (h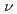 =425eV) of SPring-8. The XES and XAS spectra show a band gap between the valence and conduction bands. The valence band in the XES spectrum consists of three peaks, which extend up to
=425eV) of SPring-8. The XES and XAS spectra show a band gap between the valence and conduction bands. The valence band in the XES spectrum consists of three peaks, which extend up to  -8eV from the valence band top. The overall feature of the electronic structure of lithium amide by the XES and XAS is consistent with the band calculation. The strongly hybridized state with H 1
-8eV from the valence band top. The overall feature of the electronic structure of lithium amide by the XES and XAS is consistent with the band calculation. The strongly hybridized state with H 1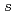 observed on the high binding energy side in the XES is discussed.
observed on the high binding energy side in the XES is discussed.

Aoki, Katsutoshi; Machida, Akihiko; Honda, Mitsunori; Hattori, Takanori; Sano, Asami; Watanuki, Tetsu; Katayama, Yoshinori; Komatsu, Kazuki*; Arima, Hiroshi; Otomo, Toshiya*; et al.
no journal, ,
Neutron diffraction measurements have revealed that LaD undergoes phase separation at high pressure with the relocation of deuterium atoms in the interstitial sites of La metal lattice. Deuterium atoms, which occupy the tetrahedral sites of the fcc metal lattice in LaD
undergoes phase separation at high pressure with the relocation of deuterium atoms in the interstitial sites of La metal lattice. Deuterium atoms, which occupy the tetrahedral sites of the fcc metal lattice in LaD , move into the empty octahedral sites at 11 GPa to form LaD and LaD
, move into the empty octahedral sites at 11 GPa to form LaD and LaD both having fcc metal lattices. Mono-hydride with an NaCl structure, which is common for mono-hydrides of transition metals, is formed in rare-earth metals for the first time. The first-principle calculations showed that LaH
both having fcc metal lattices. Mono-hydride with an NaCl structure, which is common for mono-hydrides of transition metals, is formed in rare-earth metals for the first time. The first-principle calculations showed that LaH is stable at low pressure and it undergoes a phase separation into LaH and LaH
is stable at low pressure and it undergoes a phase separation into LaH and LaH at 10 GPa, which is excellent agreement with the experimental results. Enthalpy comparison shows that unusual volume contraction in LaH
at 10 GPa, which is excellent agreement with the experimental results. Enthalpy comparison shows that unusual volume contraction in LaH than LaH
than LaH explains the phase separation phenomena. Lattice dynamics calculations on these lanthanum hydrides shed light on the detailed mechanism.
explains the phase separation phenomena. Lattice dynamics calculations on these lanthanum hydrides shed light on the detailed mechanism.

Aoki, Katsutoshi; Machida, Akihiko; Honda, Mitsunori; Hattori, Takanori; Sano, Asami; Watanuki, Tetsu; Katayama, Yoshinori; Komatsu, Kazuki*; Arima, Hiroshi; Otomo, Toshiya*; et al.
no journal, ,
Synchrotron X-ray and neutron diffraction measurements have revealed that LaD undergoes phase separation at high pressure with the relocation of deuterium atoms in the interstitial sites of La metal lattice. Synchrotron X-ray and neutron experiments were made at BL22XU, SPring-8 and a total scattering device, NOVA, J-PARC. Deuterium atoms, which occupy the tetrahedral sites of the fcc metal lattice in LaD
undergoes phase separation at high pressure with the relocation of deuterium atoms in the interstitial sites of La metal lattice. Synchrotron X-ray and neutron experiments were made at BL22XU, SPring-8 and a total scattering device, NOVA, J-PARC. Deuterium atoms, which occupy the tetrahedral sites of the fcc metal lattice in LaD , move into the empty octahedral sites at 11 GPa to form LaD and LaD
, move into the empty octahedral sites at 11 GPa to form LaD and LaD both having fcc metal lattices. Mono-hydride with an NaCl structure, which is common for mono-hydrides of transition metals, is formed in rare-earth metals for the first time. The first-principle calculations showed that LaH
both having fcc metal lattices. Mono-hydride with an NaCl structure, which is common for mono-hydrides of transition metals, is formed in rare-earth metals for the first time. The first-principle calculations showed that LaH is stable at low pressure and it undergoes a phase separation into LaH and LaH
is stable at low pressure and it undergoes a phase separation into LaH and LaH at 10 GPa, which is excellent agreement with the experimental results.
at 10 GPa, which is excellent agreement with the experimental results.
Machida, Akihiko; Honda, Mitsunori; Hattori, Takanori; Sano, Asami; Watanuki, Tetsu; Katayama, Yoshinori; Aoki, Katsutoshi; Komatsu, Kazuki*; Arima, Hiroshi; Oshita, Hidetoshi*; et al.
no journal, ,
Formation of an NaCl-type mono-deuteride LaD has been found by neutron diffraction experiments at high pressure. The NaCl-type structure has been reported for alkaline hydrides and transition metal hydrides, but not for rare-earth metal hydrides. The NaCl-type mono-hydride is formed in rare-earth metals for the first time. Lanthanum mono-deuteride is formed as a result of the phase separation of the di-deuteride under high pressure. This result suggests that the three different hydrides, mono-, di-, and tri-hydrides, with the fcc metal lattice are realized. The hydrogen atoms occupy only O-sites, only T-sites and both O-sites and T-sites in the mono-, di-, and tri-hydrides, respectively. Hence, it is expected that the H-M bonding nature is different for each hydride.
Kamakura, Nozomu; Takeda, Yukiharu; Okane, Tetsuo; Fujimori, Shinichi; Saito, Yuji; Yamagami, Hiroshi; Miyaoka, Hiroki*; Tsubota, Masami*; Ichikawa, Takayuki*; Kojima, Yoshitsugu*; et al.
no journal, ,
no abstracts in English
Kamakura, Nozomu; Yamagami, Hiroshi; Takeda, Yukiharu; Okane, Tetsuo; Saito, Yuji; Miyaoka, Hiroki*; Tsubota, Masami*; Ichikawa, Takayuki*; Kojima, Yoshitsugu*; Muro, Takayuki*; et al.
no journal, ,
In this research, the electronic states of the insulator alkali metal amide (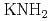 ,
, 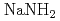 ) and alkaline earth metal amide (
) and alkaline earth metal amide (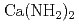 ), which are lightweight complex hydrides being considered as a high-capacity hydrogen storage material, are investigated by the soft X-ray emission (XES)and absorption spectroscopies. The sharp three peak structure commonly observed in the XES spectrum of alkali metal amides shows the localized character of the valence electrons, while the importance of the number of the amide ion is shown by the XES spectrum of
), which are lightweight complex hydrides being considered as a high-capacity hydrogen storage material, are investigated by the soft X-ray emission (XES)and absorption spectroscopies. The sharp three peak structure commonly observed in the XES spectrum of alkali metal amides shows the localized character of the valence electrons, while the importance of the number of the amide ion is shown by the XES spectrum of 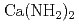 which is clearly different from that of alkali metal amide. The comparison with the band calculation clarifies the electronic sates of
which is clearly different from that of alkali metal amide. The comparison with the band calculation clarifies the electronic sates of 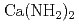 .
.

Machida, Akihiko; Honda, Mitsunori; Hattori, Takanori; Sano, Asami; Watanuki, Tetsu; Katayama, Yoshinori; Aoki, Katsutoshi; Komatsu, Kazuki*; Arima, Hiroshi; Oshita, Hidetoshi*; et al.
no journal, ,
We have investigated the pressure-induced phase separation in rare-earth metal dihydrides by synchrotron radiation X-ray diffraction and neutron diffraction experiments. Rare-earth metal hydrides exhibit the stoichiometoric dihydride and trihydride. The dihydride is a metallic while trihydride is an insulator. Recently, we have found the pressure induced phase separation in the lanthanum dihydride. This phenomenon is understood as the decomposition into the hydrogen-poor and rich phases under high pressure. However, the hydrogen occupancy and position have been unclear yet. Our neutron diffraction experiments have revealed that an NaCl-type mono-deuteride is formed as a counterpart of the deuterium-rich phase.
Kamakura, Nozomu; Takeda, Yukiharu; Yamagami, Hiroshi; Miyaoka, Hiroki*; Tsubota, Masami*; Ichikawa, Takayuki*; Kojima, Yoshitsugu*; Muro, Takayuki*; Kinoshita, Toyohiko*
no journal, ,
Metal amide has been attracted much attention as a lightweight hydride being considered for high capacity hydrogen storage materials. In this research, the electronic states of the insulator alkali metal amide (KNH , NaNH
, NaNH ) and alkaline earth metal amide (Ca(NH
) and alkaline earth metal amide (Ca(NH )
) , Mg(NH
, Mg(NH )
) ) are investigated by the soft X-ray emission spectroscopy (XES) and absorption spectroscopy (XAS) in the total fluorescence yield mode. The localized character of the valence electrons is shown by the sharp three peak structure commonly observed in the XES spectrum of alkali metal amide. The localized character of the valence electrons is shown by the sharp peak structure commonly observed in the XES spectrum of alkali metal amide. The broadening of the N 2p states by the hybridization is observed in the XES spectrum of the alkaline earth metal amide. Decomposition temperature of the metal amide is found to relate to the character of the chemical bond observed in the XAS.
) are investigated by the soft X-ray emission spectroscopy (XES) and absorption spectroscopy (XAS) in the total fluorescence yield mode. The localized character of the valence electrons is shown by the sharp three peak structure commonly observed in the XES spectrum of alkali metal amide. The localized character of the valence electrons is shown by the sharp peak structure commonly observed in the XES spectrum of alkali metal amide. The broadening of the N 2p states by the hybridization is observed in the XES spectrum of the alkaline earth metal amide. Decomposition temperature of the metal amide is found to relate to the character of the chemical bond observed in the XAS.
Aoki, Katsutoshi; Machida, Akihiko; Honda, Mitsunori; Hattori, Takanori; Sano, Asami; Watanuki, Tetsu; Katayama, Yoshinori; Komatsu, Kazuki*; Arima, Hiroshi; Otomo, Toshiya*; et al.
no journal, ,
Rare-earth metal La forms T-site occupied fcc-LaH and fully occupied fcc-LaH
and fully occupied fcc-LaH , the former is metallic and the latter is insulating. Our previous synchrotron X-ray and infrared measurements revealed that the dihydride decomposed into hydrogen-rich and hydrogen-poor phases upon compression to 11 GPa at ambient temperature; the hydrogen rich phase was identified as LaH
, the former is metallic and the latter is insulating. Our previous synchrotron X-ray and infrared measurements revealed that the dihydride decomposed into hydrogen-rich and hydrogen-poor phases upon compression to 11 GPa at ambient temperature; the hydrogen rich phase was identified as LaH but the hydrogen composition and occupation sites of the hydrogen-poor phase remained undetermined. The crystal structure of the hydrogen-poor phase was investigated for LaD
but the hydrogen composition and occupation sites of the hydrogen-poor phase remained undetermined. The crystal structure of the hydrogen-poor phase was investigated for LaD by neutron diffraction measurement with a total diffractometer NOVA at J-PARC. The formation of NaCl-type LaD as a counterpart of LaD
by neutron diffraction measurement with a total diffractometer NOVA at J-PARC. The formation of NaCl-type LaD as a counterpart of LaD by the decomposition was confirmed from the diffraction profiles. First-principle enthalpy and lattice dynamic calculations have demonstrated that the NaCl-type LaH is stabilized at high pressures.
by the decomposition was confirmed from the diffraction profiles. First-principle enthalpy and lattice dynamic calculations have demonstrated that the NaCl-type LaH is stabilized at high pressures.
Machida, Akihiko; Hattori, Takanori; Honda, Mitsunori*; Sano, Asami; Watanuki, Tetsu; Katayama, Yoshinori; Aoki, Katsutoshi; Komatsu, Kazuki*; Arima, Hiroshi*; Oshita, Hidetoshi*; et al.
no journal, ,
We have investigated the structural properties of rare-earth metal hydrides under high pressure. LaH has the CaF
has the CaF type structure in which the H atoms locate at the tetrahedral interstitial sites (T-sites) of the fcc metal lattice. Synchrotron radiation X-ray diffraction and infrared reflection experiments revealed disproportionation reaction of LaH
type structure in which the H atoms locate at the tetrahedral interstitial sites (T-sites) of the fcc metal lattice. Synchrotron radiation X-ray diffraction and infrared reflection experiments revealed disproportionation reaction of LaH into the H-poor and H-rich phases around 11 GPa. Before the disproportionation reaction, we have found that the fcc metal lattice transformed into tetragonal lattice. The ordering of the H atoms in the octahedral-sites (O-sites) causes the tetragonal distortion of LaH
into the H-poor and H-rich phases around 11 GPa. Before the disproportionation reaction, we have found that the fcc metal lattice transformed into tetragonal lattice. The ordering of the H atoms in the octahedral-sites (O-sites) causes the tetragonal distortion of LaH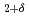 . The tetragonal transformation and successive disproportionation reaction of LaH
. The tetragonal transformation and successive disproportionation reaction of LaH would closely relate to the inter-site transfer of the H atoms between the T- and O-sites. We have performed the neutron diffraction experiments of LaD
would closely relate to the inter-site transfer of the H atoms between the T- and O-sites. We have performed the neutron diffraction experiments of LaD to investigate the change of the positions and occupancies of the hydrogen atoms under high pressure.
to investigate the change of the positions and occupancies of the hydrogen atoms under high pressure.
Machida, Akihiko; Honda, Mitsunori*; Hattori, Takanori; Sano, Asami; Watanuki, Tetsu; Katayama, Yoshinori; Aoki, Katsutoshi; Komatsu, Kazuki*; Arima, Hiroshi*; Oshita, Hidetoshi*; et al.
no journal, ,
Hydrogen atoms absorbed in a metal occupy the interstitial sites of the metal lattice. In an fcc metal lattice, each metal atom has two tetrahedral (T) and one octahedral (O) sites that can accommodate hydrogen. Rare-earth metal La forms T-site occupied LaH and fully occupied LaH
and fully occupied LaH . Previous X-ray diffraction measurements revealed the pressure-induced decomposition of an fcc-LaH
. Previous X-ray diffraction measurements revealed the pressure-induced decomposition of an fcc-LaH into H-rich and H-poor phases around 11 GPa. We performed the neutron diffraction measurements on LaD
into H-rich and H-poor phases around 11 GPa. We performed the neutron diffraction measurements on LaD , and found the formation of NaCl-type LaD as a phase separation products. We have first found the NaCl-type rare-earth metal monohydride.
, and found the formation of NaCl-type LaD as a phase separation products. We have first found the NaCl-type rare-earth metal monohydride.