Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
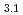 (OCOCH
(OCOCH )
)
 0.9H
0.9H O
OKozai, Naofumi; Mitamura, Hisayoshi; Fukuyama, Hiroyasu; Esaka, Fumitaka; Komarneni, S.*
Microporous and Mesoporous Materials, 89(1-3), p.123 - 131, 2006/03
Times Cited Count:12 Percentile:40.65(Chemistry, Applied)Layered transition metal hydroxide salt (LTMHS) is a group of anion-exchangeable layered compounds. Although LTMHSs have recentely attracted attention of researches on anion exchange and intercalation, very limited numbers of reports have been published on their synthesis, characteristics, and applications. This paper describes basic characteristics of a new LTMHS, nickel-copper hydroxide acetate. Hydrothermal Heating of an aqueous solution containing nickel acetate, copper acetate, and hydrogen peroxide to 150 C for 4h yielded a layered compound with an analytical composition of NiCu(OH)
C for 4h yielded a layered compound with an analytical composition of NiCu(OH)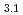 (OCOCH
(OCOCH )
) 0.9H
0.9H O. This compound does not take up Cl
O. This compound does not take up Cl and NO
and NO
 in aqueous solution but takes up multivalent anions and shows high selectivity in uptake of toxic SeO
in aqueous solution but takes up multivalent anions and shows high selectivity in uptake of toxic SeO
 and AsO
and AsO
 . This compound may find applicarion in the removal of those toxic anions form natural water and wastewater rich in Cl
. This compound may find applicarion in the removal of those toxic anions form natural water and wastewater rich in Cl and NO
and NO
 .
.
Kozai, Naofumi; Mitamura, Hisayoshi; Fukuyama, Hiroyasu; Esaka, Fumitaka; Komarneni, S.*
Journal of Materials Research, 20(11), p.2997 - 3003, 2005/11
Layered transition metal hydroxide salt (LTMHS) is a group of anion-exchangeable layered compounds. LTMHSs have lately attraced attention of researchs on anion exchange and intercalation but very limited numbers of reports have been published on their synthesis, characteristics, and application. This study reports basic properties of a layered copper hyroxide acetate synthesized by a method modified from that of the previous studies. Titration of copper acetate solution with a dilute NaOH solution to pH 6.5 and subsequent aging at 313 K yielded a layered copper hydroxide acetate. This compound has some properties similar to those of the previously known copper hydroxide acetate, Cu (OH)
(OH) (OCOCH
(OCOCH )H
)H O. The present copper hydroxide acetate is dissimilar to the previous compound in morphology, stability of bonding between the interlayer acetate ions and the matrix hydroxides, and reaction with anions in aqueous solutions.
O. The present copper hydroxide acetate is dissimilar to the previous compound in morphology, stability of bonding between the interlayer acetate ions and the matrix hydroxides, and reaction with anions in aqueous solutions.
Kozai, Naofumi; Onuki, Toshihiko; Komarneni, S.*; Kamiya, Tomihiro; Sakai, Takuro; Oikawa, Masakazu*; Sato, Takahiro
Nuclear Instruments and Methods in Physics Research B, 210, p.513 - 518, 2003/09
Times Cited Count:8 Percentile:49.71(Instruments & Instrumentation)This study examined removal of cadmium from solution by two materials with high sorptivity for cadmium: a synthetic mica named Na-4 mica and an apatite. At an initial Cd concentration of 1 10
10 M and a starting pH of 3, the distribution coefficient of Na-4 mica for Cd (8.4
M and a starting pH of 3, the distribution coefficient of Na-4 mica for Cd (8.4 10
10 ml/g) was one order of mgnitude of higher than that of apatite (8.2
ml/g) was one order of mgnitude of higher than that of apatite (8.2 10
10 ml/g). On the other thand, micro-PIXE anaysis for a mixture of equal mass of Na-4 mica and apatite contacted with Cd solution under the same conditions revealed that Cd was selectively taken up by the apatite in the mixture, while Cd was not detected on the Na-4 mica in the mixture. This result by micro-PIXE analysis is not what is expected from the above distribution coefficient values of Na-4 mica and apatite. This result can be explained by irreversibility of the uptake and the difference in kinetics of the uptake by the Na-4 mica and apatite.
ml/g). On the other thand, micro-PIXE anaysis for a mixture of equal mass of Na-4 mica and apatite contacted with Cd solution under the same conditions revealed that Cd was selectively taken up by the apatite in the mixture, while Cd was not detected on the Na-4 mica in the mixture. This result by micro-PIXE analysis is not what is expected from the above distribution coefficient values of Na-4 mica and apatite. This result can be explained by irreversibility of the uptake and the difference in kinetics of the uptake by the Na-4 mica and apatite.
Kozai, Naofumi; Onuki, Toshihiko; Komarneni, S.*
Journal of Materials Chemistry, 17(12), p.2993 - 2996, 2002/12
Here we report the extremely high and selective uptake of selenium oxyanions by a novel exchanger, Ni Zn
Zn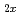 (OH)
(OH) (OCOCH
(OCOCH )
)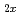 ・nH
・nH O that has brucite-type hydroxide layers with OCOCH
O that has brucite-type hydroxide layers with OCOCH anions in the interlayers. This Ni-Zn basic salt exhibited very high selectivity for Se(IV) (Kd = 9.0x10
anions in the interlayers. This Ni-Zn basic salt exhibited very high selectivity for Se(IV) (Kd = 9.0x10 ml/g with an initial Se(IV) concentration of 1x10
ml/g with an initial Se(IV) concentration of 1x10 M) in the presence of 0.1M Cl
M) in the presence of 0.1M Cl solution while the well known anionic clay, Mg
solution while the well known anionic clay, Mg Al(OH)
Al(OH) NO
NO ・nH
・nH O showed a Kd of 6.0x10
O showed a Kd of 6.0x10 ml/g under the same conditions. The uptake of Se(IV) on the Ni-Zn basic salt was found to be irreversible when treated with solutions containing 1N Cl
ml/g under the same conditions. The uptake of Se(IV) on the Ni-Zn basic salt was found to be irreversible when treated with solutions containing 1N Cl , 1N NO
, 1N NO
 , or 1N PO
, or 1N PO
 , while the Se(IV) sorbed on anionic clay was easily desorbed in a 1M Cl
, while the Se(IV) sorbed on anionic clay was easily desorbed in a 1M Cl solution. This novel exchanger also showed high Kd (2.6
solution. This novel exchanger also showed high Kd (2.6 10
10 ml/g at an initial Se concentration of 1
ml/g at an initial Se concentration of 1 10
10 M) for Se(VI) and therefore it is expected to be useful for decontamination and removal of selenium oxyanions from contaminated water.
M) for Se(VI) and therefore it is expected to be useful for decontamination and removal of selenium oxyanions from contaminated water.
Komarneni, S.*; Kozai, Naofumi; Paulus, W. J.*
Nature, 410(6830), P. 771, 2001/04
A radioactive legacy of the nuclear industry threatens many areas of the world with radioactive radium contamination from the solid and liquid waste left after extracting U from ores. Here we report that a synthetic clay of high charge is not only extremely selective for the uptake of Ra but also useful for Ra immobilization at room temperature. These results are of relevance for decontaminating the environmental and drinking water of Ra.