Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Miyata, Hokata*; Yoshida, Kenta*; Konashi, Kenji*; Du, Y.*; Kitagaki, Toru; Shobu, Takahisa; Shimada, Yusuke*
Microscopy, p.dfaf005_1 - dfaf005_10, 2025/00
Times Cited Count:0 Percentile:0.00(Microscopy) and O
and O in a reaction cell in triple quadrupole inductively coupled plasma mass spectrometry
in a reaction cell in triple quadrupole inductively coupled plasma mass spectrometryKazama, Hiroyuki; Konashi, Kenji*; Suzuki, Tatsuya*; Koyama, Shinichi; Maeda, Koji; Sekio, Yoshihiro; Onishi, Takashi; Abe, Chikage*; Shikamori, Yasuyuki*; Nagai, Yasuyoshi*
Journal of Analytical Atomic Spectrometry, 38(8), p.1676 - 1681, 2023/07
Times Cited Count:4 Percentile:63.35(Chemistry, Analytical)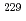 Th nuclear clock isomer determined by absolute
Th nuclear clock isomer determined by absolute  -ray energy difference
-ray energy differenceYamaguchi, Atsushi*; Muramatsu, Haruka*; Hayashi, Tasuku*; Yuasa, Naoki*; Nakamura, Keisuke; Takimoto, Misaki; Haba, Hiromitsu*; Konashi, Kenji*; Watanabe, Makoto*; Kikunaga, Hidetoshi*; et al.
Physical Review Letters, 123(22), p.222501_1 - 222501_6, 2019/11
Times Cited Count:46 Percentile:89.61(Physics, Multidisciplinary)
Uchida, Teppei; Sunaoshi, Takeo*; Konashi, Kenji*; Kato, Masato
Journal of Nuclear Materials, 452(1-3), p.281 - 284, 2014/09
Times Cited Count:11 Percentile:61.21(Materials Science, Multidisciplinary)Experiment and simulation studies on physical properties of actinide oxides have been carried out. Thermal expansion is important data to evaluate the various properties from molecular dynamics (MD) simulation. In this study, thermal expansion of PuO was evaluated by experiment and MD simulation. In the experimental study, thermal expansion of PuO
was evaluated by experiment and MD simulation. In the experimental study, thermal expansion of PuO pellet was determined by a dilatometer in the temperature range of 300-1923 K. In the MD simulation, Born-Mayer-Huggins interatomic potential with a partially ionic model and Morse potential were employed. Lattice constants of PuO
pellet was determined by a dilatometer in the temperature range of 300-1923 K. In the MD simulation, Born-Mayer-Huggins interatomic potential with a partially ionic model and Morse potential were employed. Lattice constants of PuO were evaluated in the temperature range of 300-2800 K by MD simulation, and thermal expansion was evaluated. The experimental data was good agreement with the MD simulation result. Evaluation formula for thermal expansion of PuO
were evaluated in the temperature range of 300-2800 K by MD simulation, and thermal expansion was evaluated. The experimental data was good agreement with the MD simulation result. Evaluation formula for thermal expansion of PuO in the temperature range of 300-2800 K was derived from both data.
in the temperature range of 300-2800 K was derived from both data.
Minato, Kazuo; Konashi, Kenji*; Yamana, Hajimu*; Yamanaka, Shinsuke*; Nagasaki, Shinya*; Ikeda, Yasuhisa*; Sato, Seichi*; Arita, Yuji*; Idemitsu, Kazuya*; Koyama, Tadafumi*
Proceedings of International Conference on Toward and Over the Fukushima Daiichi Accident (GLOBAL 2011) (CD-ROM), 5 Pages, 2011/12
Actinide science research is indispensable to maintain sustainable development of innovative nuclear technology. For actinide science research, special facilities with containment and radiation shields are needed to handle actinide materials. The number of facilities for actinide science research has been decreased, especially in universities, due to the high maintenance cost. J-ACTINET was established in 2008 to promote and facilitate actinide science research and to foster many of young scientists and engineers in actinide science. The research program was carried out, through which young researchers were expected to learn how to make experiments with advanced experimental tools and to broaden their horizons. The summer schools and computational science school were held to provide students and young researchers with the opportunities to come into contact with actinide science research. The overseas dispatch program was also carried out.
Osaka, Masahiko; Konashi, Kenji*; Hayashi, Hirokazu; Li, D.*; Homma, Yoshiya*; Yamamura, Tomoo*; Sato, Isamu; Miwa, Shuhei; Sekimoto, Shun*; Kubota, Takumi*; et al.
Proceedings of International Conference on Toward and Over the Fukushima Daiichi Accident (GLOBAL 2011) (CD-ROM), 5 Pages, 2011/12
Summer schools for future experts have successfully been completed under Japan Actinide Network (J-ACTINET) for the purpose of development of human resources who are expected to be engaged in every areas of actinide-research/engineering. The first summer school was held in Ibaraki-area in August 2009, followed by the second one in Kansai-area in August 2010. Two summer schools have focused on actual experiences of actinides in actinide-research fields for university students and young researchers/engineers as an introductory course of actinide-researches. Several quasi actinide-handling experiences at the actinide-research fields have attracted attentions of participants at the first school in Ibaraki-area. The actual experiments using actinides-containing solutions have been carried out at the second school in Kansai-area. Future summer schools will be held every year for the sustainable human resource development in various actinide-research fields.
 by molecular dynamics simulation
by molecular dynamics simulationUchida, Teppei; Sunaoshi, Takeo*; Kato, Masato; Konashi, Kenji*
Progress in Nuclear Science and Technology (Internet), 2, p.598 - 602, 2011/10
Interatomic potential function of UO was determined and thermal expansion, specific heat and thermal conductivity were evaluated by molecular dynamics (MD) simulation. For thermal expansion, a dilatometry was conducted and a measurement result was compared with a result of MD simulation. Thermal expansion value was in good agreement with MD simulation. Specific heat and thermal conductivity was also in good agreement with literature data when the supercell contained Schottky defects. Thermal conductivity, especially, was affected at low temperature range and decreased with increasing Schottky defect concentration. It was considered that vacancies scattered phonon vibrations. The interatomic potential function of UO
was determined and thermal expansion, specific heat and thermal conductivity were evaluated by molecular dynamics (MD) simulation. For thermal expansion, a dilatometry was conducted and a measurement result was compared with a result of MD simulation. Thermal expansion value was in good agreement with MD simulation. Specific heat and thermal conductivity was also in good agreement with literature data when the supercell contained Schottky defects. Thermal conductivity, especially, was affected at low temperature range and decreased with increasing Schottky defect concentration. It was considered that vacancies scattered phonon vibrations. The interatomic potential function of UO was considered usable.
was considered usable.
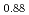 Pu
Pu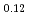 )O
)O and (U
and (U Pu
Pu )O
)O at high temperatures of 1673-1873 K
at high temperatures of 1673-1873 KKato, Masato; Takeuchi, Kentaro; Uchida, Teppei; Sunaoshi, Takeo*; Konashi, Kenji*
Journal of Nuclear Materials, 414(2), p.120 - 125, 2011/07
Times Cited Count:23 Percentile:83.11(Materials Science, Multidisciplinary)Many studies on oxygen potentials have been reported, but their data were scattered and the data at high temperatures are limited. In this work, the oxygen potential of (U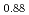 Pu
Pu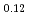 )O
)O and (U
and (U Pu
Pu )O
)O was measured at high temperatures of 1673-1873 K using gas equilibrium method using thermo-gravimetry. The influence of Pu addition on the oxygen potential of MOX was discussed. The oxygen potential and the O/M ratio were decided by in-situ analysis. The oxygen partial pressure was adjusted by controlling the ratio of
was measured at high temperatures of 1673-1873 K using gas equilibrium method using thermo-gravimetry. The influence of Pu addition on the oxygen potential of MOX was discussed. The oxygen potential and the O/M ratio were decided by in-situ analysis. The oxygen partial pressure was adjusted by controlling the ratio of 
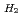 /
/
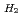 O in the flowing gas atmosphere, and the oxygen potential was determined. The oxygen potentials measured by the point defect model. The deviation x varied with the relation of in the near stoichiometric composition region. The oxygen potential increased with increasing Pu content. The values of stoichiometric MOX containing 12% and 30%Pu were determined to be -334 kJ/mol and -296 kJ/mol, respectively, at 1773 K.
O in the flowing gas atmosphere, and the oxygen potential was determined. The oxygen potentials measured by the point defect model. The deviation x varied with the relation of in the near stoichiometric composition region. The oxygen potential increased with increasing Pu content. The values of stoichiometric MOX containing 12% and 30%Pu were determined to be -334 kJ/mol and -296 kJ/mol, respectively, at 1773 K.
Minato, Kazuo; Konashi, Kenji*; Fujii, Toshiyuki*; Uehara, Akihiro*; Nagasaki, Shinya*; Otori, Norikazu*; Tokunaga, Yo; Kambe, Shinsaku
IOP Conference Series; Materials Science and Engineering, 9, p.012018_1 - 012018_7, 2010/05
Times Cited Count:0 Percentile:0.00(Chemistry, Inorganic & Nuclear)Basic research in actinide chemistry and physics is indispensable to maintain sustainable development of innovative nuclear technology. Actinides, especially minor actinides of americium and curium, need to be handled in special facilities with containment and radiation shields. To promote and facilitate the actinide research, close cooperation with the facilities and sharing of technical and scientific information must be very important and effective. A three-year-program "Basic actinide chemistry and physics research in close cooperation with hot laboratories", ACTILAB, was started to form the bases of sustainable development of innovative nuclear technology. In this program, researches on actinide solid-state physics, solution chemistry and solid-liquid interface chemistry are made using four main facilities in Japan in close cooperation with each other, where basic experiments with transuranium elements can be made. The  O-NMR measurements were performed on (Pu
O-NMR measurements were performed on (Pu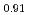 Am
Am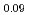 )O
)O to study the electronic state and the chemical behaviour of Am and Cm ions in electrolyte solutions was studied by distribution experiments.
to study the electronic state and the chemical behaviour of Am and Cm ions in electrolyte solutions was studied by distribution experiments.
Kato, Masato; Komeno, Akira; Uno, Hiroki*; Sugata, Hiromasa*; Nakae, Nobuo; Konashi, Kenji*; Kashimura, Motoaki
Journal of Nuclear Materials, 393(1), p.134 - 140, 2009/08
Times Cited Count:44 Percentile:92.68(Materials Science, Multidisciplinary)In plutonium compounds, the lattice parameter increases due to self-radiation damage by  -decay of plutonium isotopes. The lattice parameter change and its thermal recovery in plutonium and uranium mixed dioxide (MOX) were studied. The lattice parameter for samples of MOX powders and pellets that had been left in the air for up to 32 years was measured. The lattice parameter increased and was saturated at about 0.29%. The change in lattice parameter was formulated as a function of self-radiation dose. Three stages in the thermal recovery of the damage were observed in temperature ranges of below 673K, 673-1073K and above 1073K. The activation energies in each recovery stage were estimated to be 0.12 eV, 0.73 eV and 1.2 eV, respectively, and the corresponding mechanism for each stage was considered to be the recovery of the anion Frenkel defect, the cation Frenkel defect and a defect connected with helium, respectively.
-decay of plutonium isotopes. The lattice parameter change and its thermal recovery in plutonium and uranium mixed dioxide (MOX) were studied. The lattice parameter for samples of MOX powders and pellets that had been left in the air for up to 32 years was measured. The lattice parameter increased and was saturated at about 0.29%. The change in lattice parameter was formulated as a function of self-radiation dose. Three stages in the thermal recovery of the damage were observed in temperature ranges of below 673K, 673-1073K and above 1073K. The activation energies in each recovery stage were estimated to be 0.12 eV, 0.73 eV and 1.2 eV, respectively, and the corresponding mechanism for each stage was considered to be the recovery of the anion Frenkel defect, the cation Frenkel defect and a defect connected with helium, respectively.
Kato, Masato; Morimoto, Kyoichi; Tamura, Tetsuya*; Sunaoshi, Takeo*; Konashi, Kenji*; Aono, Shigenori; Kashimura, Motoaki
Journal of Nuclear Materials, 389(3), p.416 - 419, 2009/06
Times Cited Count:12 Percentile:60.72(Materials Science, Multidisciplinary)Plutonium and uranium mixed oxide (MOX) has been developed to use as a core fuel of the fast reactor. The oxygen to metal ratio (O/M) of the MOX fuel is an important parameter to control the FCCI. The oxygen potential and the oxygen diffusion coefficient of the MOX are essential data to understand the oxygen behaviour in MOX. The oxygen potentials of the MOX were measured with accuracy as a function of O/M and temperatures in the previous work. In this work the oxygen chemical diffusion coefficient in (Pu U
U )O
)O and (Pu
and (Pu U
U )O
)O were investigated using thermo gravimetric technique. The kinetics of the reduction processes of (Pu
were investigated using thermo gravimetric technique. The kinetics of the reduction processes of (Pu U
U )O
)O and (Pu
and (Pu U
U )O
)O were measured by TG-DTA method. The oxygen chemical diffusion coefficients have been estimated from the reduction curves. It was concluded that the oxygen chemical diffusion coefficient in (Pu
were measured by TG-DTA method. The oxygen chemical diffusion coefficients have been estimated from the reduction curves. It was concluded that the oxygen chemical diffusion coefficient in (Pu U
U )O
)O is a smaller than that of (Pu
is a smaller than that of (Pu U
U )O
)O .
.
 Pu
Pu )O
)O and (U
and (U Pu
Pu )O
)O based on point defect chemistry
based on point defect chemistryKato, Masato; Konashi, Kenji*; Nakae, Nobuo
Journal of Nuclear Materials, 389(1), p.164 - 169, 2009/05
Times Cited Count:27 Percentile:83.84(Materials Science, Multidisciplinary)Stoichiometries in (U Pu
Pu )O
)O and (U
and (U Pu
Pu )O
)O were analyzed from the experimental data of oxygen potential based on point defect chemistry. The relation between the deviation x from stoichimetric composition and oxygen partial pressure were evaluated. The concentrations of the point defects in MOX were estimated from the measured data of oxygen potential as functions of temperature and using Kr
were analyzed from the experimental data of oxygen potential based on point defect chemistry. The relation between the deviation x from stoichimetric composition and oxygen partial pressure were evaluated. The concentrations of the point defects in MOX were estimated from the measured data of oxygen potential as functions of temperature and using Kr ger-Vink diagram. The analysis results showed that x was proportional to near stoichiometric region of both (U
ger-Vink diagram. The analysis results showed that x was proportional to near stoichiometric region of both (U Pu
Pu )O
)O and (U
and (U Pu
Pu )O
)O , which suggested that intrinsic ionization was dominant defect. The model to calculate oxygen potential was derived and represented the experimental data with precision, and thermodynamic data.
, which suggested that intrinsic ionization was dominant defect. The model to calculate oxygen potential was derived and represented the experimental data with precision, and thermodynamic data.
 by the first-principles and lattice vibration
by the first-principles and lattice vibrationMinamoto, Satoshi*; Kato, Masato; Konashi, Kenji*; Kawazoe, Yoshiyuki*
Journal of Nuclear Materials, 385(1), p.18 - 20, 2009/03
Times Cited Count:33 Percentile:88.11(Materials Science, Multidisciplinary)Plutonium dioxide (PuO ) is the key compounds which will take effect the thermal properties for MOX fuels. But due to the lack of experimental data on plutonium dioxide, computational thermodynamics data were not established. In recently, the coupling of first-principle calculation and lattice dynamics theory, computational thermodynamics data could be obtained numerically. We applied first principle plane-wave calculation and lattice dynamics theory to estimate thermal properties of plutonium oxide for perfect crystal. Total energy calculation for perfect crystal reproduced experimental lattice parameter well. And after phonon dispersion calculation for plutonium dioxide, contribution of lattice vibration to thermal properties was investigated.
) is the key compounds which will take effect the thermal properties for MOX fuels. But due to the lack of experimental data on plutonium dioxide, computational thermodynamics data were not established. In recently, the coupling of first-principle calculation and lattice dynamics theory, computational thermodynamics data could be obtained numerically. We applied first principle plane-wave calculation and lattice dynamics theory to estimate thermal properties of plutonium oxide for perfect crystal. Total energy calculation for perfect crystal reproduced experimental lattice parameter well. And after phonon dispersion calculation for plutonium dioxide, contribution of lattice vibration to thermal properties was investigated.

Kato, Masato; Konashi, Kenji*
Journal of Nuclear Materials, 385(1), p.117 - 121, 2009/03
Times Cited Count:47 Percentile:93.67(Materials Science, Multidisciplinary)Homogeneous MOX fuel containing minor actinide elements has been developed as a fuel of an advanced fast reactor. In this work, the solid solution of (U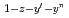 Pu
Pu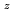 Am
Am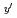 Np
Np 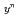 )O
)O (z=0-1, y'=0-0.12, y"=0-0.07) were investigated by X ray diffraction measurement, and database for the lattice parameter was updated. A model to calculate the lattice parameter was derived from the database using radii of ion composing fluorite structure. The radii of ion were estimated from the lattice parameters measured in this work. The model represented the experimental data within standard deviation of
(z=0-1, y'=0-0.12, y"=0-0.07) were investigated by X ray diffraction measurement, and database for the lattice parameter was updated. A model to calculate the lattice parameter was derived from the database using radii of ion composing fluorite structure. The radii of ion were estimated from the lattice parameters measured in this work. The model represented the experimental data within standard deviation of  =
= 0.025%.
0.025%.
Kato, Masato; Tamura, Tetsuya*; Konashi, Kenji*
Journal of Nuclear Materials, 385(2), p.419 - 423, 2009/03
Times Cited Count:25 Percentile:82.03(Materials Science, Multidisciplinary)Oxygen potentials of homogenous (Pu U
U )O
)O and (Np
and (Np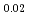 Am
Am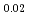 Pu
Pu U
U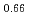 )O
)O which have been developed as a fuel of fast breeder reactors were measured at temperatures of 1473 - 1623 K by gas equilibrium technique using Ar/H
which have been developed as a fuel of fast breeder reactors were measured at temperatures of 1473 - 1623 K by gas equilibrium technique using Ar/H /H
/H O gas mixture. Oxygen potentials of (Pu
O gas mixture. Oxygen potentials of (Pu U
U )O
)O measured in this work were about 25 kJ/mol lower than those of (Pu
measured in this work were about 25 kJ/mol lower than those of (Pu U
U )O
)O and were consistent with the value calculated by Besmenn and Lindemer's model. Those of (Np
and were consistent with the value calculated by Besmenn and Lindemer's model. Those of (Np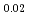 Am
Am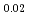 Pu
Pu U
U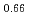 )O
)O were slightly higher than those of MOX without minor actinides.
were slightly higher than those of MOX without minor actinides.
Kato, Masato; Morimoto, Kyoichi; Nakamichi, Shinya; Sugata, Hiromasa*; Konashi, Kenji*; Kashimura, Motoaki; Abe, Tomoyuki
Nihon Genshiryoku Gakkai Wabun Rombunshi, 7(4), p.420 - 428, 2008/12
The melting temperatures of MOX for fast reactor fuel were investigated as functions of Pu content, Am content and oxygen-to-metal (O/M) ratio using thermal arrest technique. Rhenium inner was used for the measurement to prevent the reaction between the sample and capsule materials. The solidus temperatures decreased with increasing Pu and Am content and increased with decreasing O/M ratio. It is considered that the maximum temperature in U-Pu-O system varies in hypostoichiometric composition region. The melting temperatures were evaluated by ideal solid solution model in UO -PuO
-PuO -AmO
-AmO -PuO
-PuO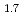 system, and the model was derived for calculating solidus and liquidus temperature. The derived model reproduced the experimental data with
system, and the model was derived for calculating solidus and liquidus temperature. The derived model reproduced the experimental data with  25 K.
25 K.
 -phase zirconium hydrides and deuterides
-phase zirconium hydrides and deuteridesTsuchiya, Bun*; Yasuda, Ryo; Teshigawara, Makoto; Konashi, Kenji*; Nagata, Shinji*; Shikama, Tatsuo*; Yamawaki, Michio*
Journal of Nuclear Materials, 376(1), p.60 - 65, 2008/05
Times Cited Count:2 Percentile:16.35(Materials Science, Multidisciplinary)Distributions of hydrogen isotope concentrations in e-phase zirconium hydrides and deuterides ( -ZrHx and
-ZrHx and  -ZrDx: 1.8
-ZrDx: 1.8  x
x  2.0) were investigated by neutron radiography (NRG). The NRG images of the thermal neutron transmission and backscattering revealed hydrogen concentration dependence and isotope differences. The thermal neutron mass attenuation coefficients in relation to the hydrogen isotope concentrations were determined from the transmission NRG images. The results showed the isotope effects of the thermal neutron mass attenuation coefficients for
2.0) were investigated by neutron radiography (NRG). The NRG images of the thermal neutron transmission and backscattering revealed hydrogen concentration dependence and isotope differences. The thermal neutron mass attenuation coefficients in relation to the hydrogen isotope concentrations were determined from the transmission NRG images. The results showed the isotope effects of the thermal neutron mass attenuation coefficients for  -ZrHx to be about 6-9 times higher than those for
-ZrHx to be about 6-9 times higher than those for  -ZrDx. The neutron scattering processes for transmission and backscattering NRG images of
-ZrDx. The neutron scattering processes for transmission and backscattering NRG images of  -ZrHx and
-ZrHx and  -ZrDx were also analyzed using a general Monte Carlo neutron-particle transport (MCNP) code.
-ZrDx were also analyzed using a general Monte Carlo neutron-particle transport (MCNP) code.
Kato, Masato; Morimoto, Kyoichi; Sugata, Hiromasa*; Konashi, Kenji*; Kashimura, Motoaki; Abe, Tomoyuki
Journal of Alloys and Compounds, 452(1), p.48 - 53, 2008/03
Times Cited Count:32 Percentile:78.13(Chemistry, Physical)Plutonium and uranium mixed oxide has been developed as a fuel of a fast reactor. The maximum temperature of the fuel pellet is limited within a design criterion to prevent fuel melting. So, the melting points of the mixed oxide have been investigated since the development of fast reactor started. However the measured data are limited. In this work, the melting points of (U1-yPuy)O (y: 0, 0.12, 0.2, 0.3, 0.4) were measured by the thermal arrest method. The evaluated melting point of this study underestimates in case of MOX with high Pu contents of 30% and 40%. The solidus of UO
(y: 0, 0.12, 0.2, 0.3, 0.4) were measured by the thermal arrest method. The evaluated melting point of this study underestimates in case of MOX with high Pu contents of 30% and 40%. The solidus of UO , (Pu
, (Pu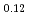 U
U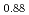 )O
)O and (Pu
and (Pu U
U )O
)O were determined to be 3128K, 3077K and 3052K, respectively. The solidus temperature of hypostoichiometric MOX slightly increased with decreasing O/M.
were determined to be 3128K, 3077K and 3052K, respectively. The solidus temperature of hypostoichiometric MOX slightly increased with decreasing O/M.
 -PuO
-PuO system
systemKato, Masato; Morimoto, Kyoichi; Sugata, Hiromasa*; Konashi, Kenji*; Kashimura, Motoaki; Abe, Tomoyuki
Journal of Nuclear Materials, 373(1-3), p.237 - 245, 2008/02
Times Cited Count:64 Percentile:96.04(Materials Science, Multidisciplinary)The melting of plutonium and uranium mixed oxide (MOX) containing Pu of more than 30% was investigated using a tungsten capsule and a rhenium inner capsule. In the conventional measurement of MOX in the tungsten capsule, a liquid phase of tungsten and plutonium oxide appeared in the MOX during melting. This liquid phase was found to have an effect on the measurement of melting point. Therefore the rhenium inner capsule was used to avoid the effect. The solidus and liquidus temperatures in the UO -PuO
-PuO system were decided from the MOX data measured using the rhenium capsule, and the effect of the Am content on the solidus temperature was evaluated. The variation of the solidus and liquidus temperatures in the UO
system were decided from the MOX data measured using the rhenium capsule, and the effect of the Am content on the solidus temperature was evaluated. The variation of the solidus and liquidus temperatures in the UO -PuO
-PuO -AmO
-AmO ternary system was represented to an accuracy of
ternary system was represented to an accuracy of  =
= 9K and
9K and  =
= 16K, respectively, by the ideal solution model.
16K, respectively, by the ideal solution model.
Kato, Masato; Morimoto, Kyoichi; Sugata, Hiromasa*; Konashi, Kenji*; Kashimura, Motoaki; Abe, Tomoyuki
Transactions of the American Nuclear Society, 96(1), p.193 - 194, 2007/06
Melting point of a nuclear fuel is one of the important physical properties for its development, because it limits maximum temperature of the fuel during operation. A rhenium inner capsule was used to prevent the reaction with capsule for measuring melting points of MOX. In this work melting points of MOX with 40% and 46%Pu were investigated as a function of an O/M ratio using Re inner, and the effect of the O/M ratio on the melting points was evaluated. The solidus and liquidus temperatures in (Pu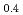 U
U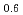 )O
)O and (Pu
and (Pu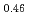 U
U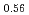 )O
)O were measured by thermal arrest method. It was observed that the melting points in the both samples increased with a decrease of the O/M from 2.00, and their data were 50-100K higher than existing data measured in previous works which were measured with W capsule.
were measured by thermal arrest method. It was observed that the melting points in the both samples increased with a decrease of the O/M from 2.00, and their data were 50-100K higher than existing data measured in previous works which were measured with W capsule.