Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Ni
Ni Mn
Mn Co
Co O
O in a lithium-ion battery
in a lithium-ion batteryKonishi, Hiroaki*; Hirano, Tatsumi*; Takamatsu, Daiko*; Gunji, Akira*; Feng, X.*; Furutsuki, Sho*; Okumura, Takafumi*; Terada, Shohei*; Tamura, Kazuhisa
Journal of Solid State Chemistry, 262, p.294 - 300, 2018/06
Times Cited Count:10 Percentile:47.54(Chemistry, Inorganic & Nuclear)The potential in each state of charge (SOC) during charging of Li Ni
Ni Mn
Mn Co
Co O
O is higher than that during discharging. To clarify the effect of chargedischarge operating conditions on the electrochemical reaction, Li
is higher than that during discharging. To clarify the effect of chargedischarge operating conditions on the electrochemical reaction, Li Ni
Ni Mn
Mn Co
Co O
O was charged and discharged under various charge-discharge operating ranges, and OCP, crystal structure, and oxidation states of the ransition metals were evaluated by electrochemical measurement, XRD, and XAFS. These results indicate that OCP, lattice parameters, and oxidation states of the transition metals of Li
was charged and discharged under various charge-discharge operating ranges, and OCP, crystal structure, and oxidation states of the ransition metals were evaluated by electrochemical measurement, XRD, and XAFS. These results indicate that OCP, lattice parameters, and oxidation states of the transition metals of Li Ni
Ni Mn
Mn Co
Co O
O in each SOC are not constant. The XRD results indicate that two phases, namely, LiNi
in each SOC are not constant. The XRD results indicate that two phases, namely, LiNi Mn
Mn Co
Co O
O -like and Li
-like and Li MnO
MnO -like, exist in Li
-like, exist in Li Ni
Ni Mn
Mn Co
Co O
O .
.
 Ni
Ni Mn
Mn Co
Co O
O used for lithium ion batteries
used for lithium ion batteriesKonishi, Hiroaki*; Hirano, Tatsumi*; Takamatsu, Daiko*; Gunji, Akira*; Feng, X.*; Furutsuki, Sho*; Okumura, Takafumi*; Terada, Shohei*; Tamura, Kazuhisa
Journal of Solid State Chemistry, 258, p.225 - 231, 2018/02
Times Cited Count:9 Percentile:43.89(Chemistry, Inorganic & Nuclear)Li Ni
Ni Mn
Mn Co
Co O
O is known as one of the cathode electrode material for Li ion batteries and its structure during charge and discharge process was investigated using electrochemical method and X-ray diffraction. It was found that in the charge process the structure changes in the order of Li
is known as one of the cathode electrode material for Li ion batteries and its structure during charge and discharge process was investigated using electrochemical method and X-ray diffraction. It was found that in the charge process the structure changes in the order of Li MnO
MnO , LiNi
, LiNi Mn
Mn Co
Co O
O , and Li
, and Li MnO
MnO . On the other hand, in the discharge process, the structure changes in the order of Li
. On the other hand, in the discharge process, the structure changes in the order of Li MnO
MnO and LiNi
and LiNi Mn
Mn Co
Co O
O .
.
 Mn
Mn O
O during electrochemical reaction on epitaxial thin-film electrodes characterized by
during electrochemical reaction on epitaxial thin-film electrodes characterized by  X-ray scattering
X-ray scatteringSakamoto, Kazuyuki*; Hirayama, Masaaki*; Konishi, Hiroaki*; Sonoyama, Noriyuki*; Dupr , N.*; Guyomard, D.*; Tamura, Kazuhisa; Mizuki, Junichiro; Kanno, Ryoji*
, N.*; Guyomard, D.*; Tamura, Kazuhisa; Mizuki, Junichiro; Kanno, Ryoji*
Physical Chemistry Chemical Physics, 12(15), p.3815 - 3823, 2010/04
Times Cited Count:34 Percentile:73.64(Chemistry, Physical)Surface and bulk structural changes of LiNi Mn
Mn O
O were investigated during electrochemical reaction using synchrotron X-ray scattering and a restricted reaction plane consisting of two dimensional epitaxial-film electrodes. The changes in bulk structure confirmed lithium diffusion through the (110) surface, which was perpendicular to the two-dimensional (2D) edges of the layered structure. No (de)intercalation reaction was observed through the (003) surface at voltages of 3.0-5.0 V. However, intercalation did proceed through the (003) plane below 3.0 V, indicating unusual three-dimensional (3D) lithium diffusion in the over-lithiated 2D structure. During the electrochemical process, the surface of the electrode showed different structure changes from those of the bulk structure. The reaction echanism of the intercalation electrodes for lithium batteries is discussed on the basis of surface and bulk structural changes.
were investigated during electrochemical reaction using synchrotron X-ray scattering and a restricted reaction plane consisting of two dimensional epitaxial-film electrodes. The changes in bulk structure confirmed lithium diffusion through the (110) surface, which was perpendicular to the two-dimensional (2D) edges of the layered structure. No (de)intercalation reaction was observed through the (003) surface at voltages of 3.0-5.0 V. However, intercalation did proceed through the (003) plane below 3.0 V, indicating unusual three-dimensional (3D) lithium diffusion in the over-lithiated 2D structure. During the electrochemical process, the surface of the electrode showed different structure changes from those of the bulk structure. The reaction echanism of the intercalation electrodes for lithium batteries is discussed on the basis of surface and bulk structural changes.
 Mn
Mn O
O
Sakamoto, Kazuyuki*; Konishi, Hiroaki*; Sonoyama, Noriyuki*; Yamada, Atsuo*; Tamura, Kazuhisa; Mizuki, Junichiro; Kanno, Ryoji*
Journal of Power Sources, 174(2), p.678 - 682, 2007/12
Times Cited Count:25 Percentile:59.38(Chemistry, Physical)Structure changes of LiNi Mn
Mn O
O were detected at the electrode/electrolyte interface of lithium cell using synchrotron X-ray scattering and two-dimensional model electrodes. The electrodes were constructed by an epitaxial film of LiNi
were detected at the electrode/electrolyte interface of lithium cell using synchrotron X-ray scattering and two-dimensional model electrodes. The electrodes were constructed by an epitaxial film of LiNi Mn
Mn O
O synthesized by pulsed laser deposition (PLD) method. The orientation of the film depends on the substrate plane; the 2D layer of LiNi
synthesized by pulsed laser deposition (PLD) method. The orientation of the film depends on the substrate plane; the 2D layer of LiNi Mn
Mn O
O is parallel to the SrTiO
is parallel to the SrTiO (1 1 0) substrate ((1 1 0) LiNi
(1 1 0) substrate ((1 1 0) LiNi Mn
Mn O
O //(1 1 0) SrTiO
//(1 1 0) SrTiO ), while the 2D layer is perpendicular to the SrTiO
), while the 2D layer is perpendicular to the SrTiO (1 1 1) substrate ((0 0 3) LiNi
(1 1 1) substrate ((0 0 3) LiNi Mn
Mn O
O //(1 1 1) SrTiO
//(1 1 1) SrTiO ). The
). The  X-ray diffraction of LiNi
X-ray diffraction of LiNi Mn
Mn O
O (0 0 3) confirmed three-dimensional lithium diffusion through the two-dimensional transition meal layers. The intercalation reaction of LiNi
(0 0 3) confirmed three-dimensional lithium diffusion through the two-dimensional transition meal layers. The intercalation reaction of LiNi Mn
Mn O
O will be discussed.
will be discussed.
Nakamura, Yukiharu; Tsutsui, Hiroaki*; Takei, Nahoko*; Sakamoto, Yoshiteru; Fujita, Takaaki; Sugihara, Masayoshi; Ozeki, Takahisa; Tobita, Kenji; Konishi, Satoshi; Iio, Shunji*; et al.
Europhysics Conference Abstracts, 27A, 4 Pages, 2003/00
no abstracts in English
Ikemoto, Megumi*; Somekawa, Jun*; Neki, Arata*; Konishi, Ren*; Nakashima, Ryota*; Okutsu, Kenichi*; Kino, Yasushi*; Yamashita, Takuma*; Okada, Shinji*; Sato, Motoyasu*; et al.
no journal, ,
no abstracts in English
Konishi, Ren*; Okutsu, Kenichi*; Kino, Yasushi*; Sasaki, Kyosuke*; Nakashima, Ryota*; Yamashita, Takuma*; Miyashita, Konan*; Yasuda, Kazuhiro*; Okada, Shinji*; Sato, Motoyasu*; et al.
no journal, ,
Muon catalyzed fusion ( CF) is a cyclic reaction where a negatively charged muon itself acts like a catalyst of nuclear fusion between hydrogen isotopes. In this work, we used PHITS code to simulate the behavior of the low-energy muon in a thin layer of the solid hydrogen.
CF) is a cyclic reaction where a negatively charged muon itself acts like a catalyst of nuclear fusion between hydrogen isotopes. In this work, we used PHITS code to simulate the behavior of the low-energy muon in a thin layer of the solid hydrogen.
Konishi, Ren*; Okutsu, Kenichi*; Kino, Yasushi*; Sasaki, Kyosuke*; Nakashima, Ryota*; Miyashita, Konan*; Yasuda, Kazuhiro*; Yamashita, Takuma*; Okada, Shinji*; Sato, Motoyasu*; et al.
no journal, ,
When muons are injected into a deuterium thin film target, muon molecules are formed. The muons released after intramolecular fusion (recycling muons) are important for the development of slow muon beams. In this study, corresponding to an experiment in which recycling muons are transported using a coaxial transport tube, the energy distribution of scattered muons, muons after deceleration, and background radiation due to bremsstrahlung by decay electrons and neutrons are analyzed by numerical simulations.
Konishi, Ren*; Okutsu, Kenichi*; Kino, Yasushi*; Sasaki, Kyosuke*; Nakashima, Ryota*; Miyashita, Konan*; Yasuda, Kazuhiro*; Yamashita, Takuma*; Okada, Shinji*; Sato, Motoyasu*; et al.
no journal, ,
We are attempting to observe regenerative muons emitted from the surface of a solid hydrogen thin film by muon-catalyzed fusion by irradiating the film with muons that have the same charge as electrons and 207 times the mass of electrons. The main background factors in detecting regenerative muons are scattered muons from the accelerator, which are slowed down to the same level as regenerative muons by the target, and bremsstrahlung generated by the components of the device. The results show that there is little scattering within the solid hydrogen, and that the dominant slowing down process is at the Al foil upstream of the solid hydrogen target. The energy distribution of Bremsstrahlung at the X-ray detection position will be reported.
Ikemoto, Megumi*; Somekawa, Jun*; Neki, Arata*; Konishi, Ren*; Nakashima, Ryota*; Okutsu, Kenichi*; Kino, Yasushi*; Yamashita, Takuma*; Okada, Shinji*; Sato, Motoyasu*; et al.
no journal, ,
We have been studying on muon beam quality improvement by moderating 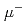 generated by an accelerator with a thin Si film, and then decelerating and focusing the beam in an electrostatic field. In this study, numerical simulation of an experiment in which
generated by an accelerator with a thin Si film, and then decelerating and focusing the beam in an electrostatic field. In this study, numerical simulation of an experiment in which 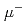 of a few MeV is injected into a 0.5~mm thick Si plate and
of a few MeV is injected into a 0.5~mm thick Si plate and 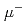 , which is decelerated to a few keV, is extracted electrostatically is performed using charged particle orbit software (SIMION). The flight time to the end of the transport tube and the transport efficiency change with a slight shift of the muon launch position, suggesting that the muon transport process is sensitive to the initial conditions.
, which is decelerated to a few keV, is extracted electrostatically is performed using charged particle orbit software (SIMION). The flight time to the end of the transport tube and the transport efficiency change with a slight shift of the muon launch position, suggesting that the muon transport process is sensitive to the initial conditions.