Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
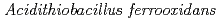
Kanao, Tadayoshi*; Kosaka, Megumi*; Yoshida, Kyoya*; Nakayama, Hisayuki*; Tamada, Taro; Kuroki, Ryota; Yamada, Hidenori; Takada, Jun*; Kamimura, Kazuo*
Acta Crystallographica Section F, 69(6), p.692 - 694, 2013/06
Times Cited Count:10 Percentile:59.68(Biochemical Research Methods)Tetrathionate hydrolase (4THase) from the iron- and sulfur-oxidizing bacterium 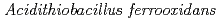 catalyses the disproportionate hydrolysis of tetrathionate to elemental sulfur, thiosulfate and sulfate. The gene encoding 4THase (
catalyses the disproportionate hydrolysis of tetrathionate to elemental sulfur, thiosulfate and sulfate. The gene encoding 4THase (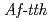 ) was expressed as inclusion bodies in recombinant
) was expressed as inclusion bodies in recombinant  . Recombinant
. Recombinant 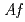 -Tth was activated by refolding under acidic conditions and was then purified to homogeneity. The recombinant protein was crystallized in 20 m
-Tth was activated by refolding under acidic conditions and was then purified to homogeneity. The recombinant protein was crystallized in 20 m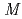 glycine buffer pH 10 containing 50 m
glycine buffer pH 10 containing 50 m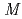 sodium chloride and 33%(
sodium chloride and 33%(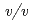 ) PEG 1000 using the hanging-drop vapour-diffusion method. The crystal was a hexagonal cylinder with dimensions of 0.2
) PEG 1000 using the hanging-drop vapour-diffusion method. The crystal was a hexagonal cylinder with dimensions of 0.2  0.05
0.05  0.05 mm. X-ray crystallographic analysis showed that the crystal diffracted to 2.15
0.05 mm. X-ray crystallographic analysis showed that the crystal diffracted to 2.15  resolution and belongs to space group
resolution and belongs to space group  3
3 or
or  3
3 , with unit-cell parameters
, with unit-cell parameters  =
=  = 92.1,
= 92.1,  = 232.6
= 232.6  .
.
Yamada, Hidenori*; Tamada, Taro; Kosaka, Megumi*; Miyata, Kohei*; Fujiki, Shinya*; Tano, Masaru*; Moriya, Masayuki*; Yamanishi, Mamoru*; Honjo, Eijiro; Tada, Horiko*; et al.
Protein Science, 16(7), p.1389 - 1397, 2007/07
Times Cited Count:39 Percentile:59.61(Biochemistry & Molecular Biology)In an attempt to control protein incorporation in a crystal lattice, a leucine zipper-like hydrophobic interface (comprising four leucine residues) was introduced into a helical region (helix 2) of the human pancreatic ribonuclease 1 (RNase 1) that was predicted to form a suitable crystallization interface. Although crystallization of wild type RNase 1 has not yet been reported, the RNase 1 mutant having four leucines (4L-RNase 1) was successfully crystallized under several different conditions. The crystal structures were subsequently determined by X-ray crystallography by molecular replacement using the structure of bovine RNase A. The overall structure of 4L-RNase 1 is quite similar to that of the bovine RNase A, and the introduced leucine residues formed the designed crystal interface. To further characterize the role of the introduced leucine residues in crystallization of RNase 1, the number of leucines was reduced to three or two (3L- and 2L-RNase 1, respectively). Both mutants crystallized and a similar hydrophobic interface as in 4L-RNase 1 was observed. A related approach to engineer crystal contacts at helix 3 of RNase 1 (N4L-RNase 1) was also evaluated. N4L-RNase 1 also successfully crystallized, and formed the expected hydrophobic packing interface. These results suggest that appropriate introduction of a leucine zipper-like hydrophobic interface can promote intra molecular symmetry for more efficient protein crystallization in crystal lattice engineering efforts.
Kosaka, Megumi*; Kanao, Tadayoshi*; Nakayama, Hisayuki*; Yoshida, Kyoya*; Kamimura, Kazuo*; Takada, Jun*; Yamada, Hidenori; Tamada, Taro; Okazaki, Nobuo*; Kuroki, Ryota
no journal, ,
no abstracts in English