Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Morita, Yasuji; Kawata, Yoshihisa*; Mineo, Hideaki; Koshino, Nobuyoshi*; Asanuma, Noriko*; Ikeda, Yasuhisa*; Yamasaki, Kazuhiko*; Chikazawa, Takahiro*; Tamaki, Yoshihisa*; Kikuchi, Toshiaki*
Journal of Nuclear Science and Technology, 44(3), p.354 - 360, 2007/03
Times Cited Count:16 Percentile:70.99(Nuclear Science & Technology)Precipitation behavior of Pu and other transuranium elements with N-cyclohexyl-2-pyrrolidone (NCP) has been examined to develop a simple reprocessing based only on precipitation method. From HNO solutions containing only Pu, both Pu(VI) and Pu(IV) were precipitated with NCP, but they required more NCP than in the U(VI) precipitation. Selective U(VI) precipitation from HNO
solutions containing only Pu, both Pu(VI) and Pu(IV) were precipitated with NCP, but they required more NCP than in the U(VI) precipitation. Selective U(VI) precipitation from HNO solution containing U(VI) and Pu(IV) was achieved by stirring the solution for sufficient time after addition of NCP with ratio of [NCP]/[U]=1.4. Addition of an enough amount of NCP to U(VI)-Pu(VI) or U(VI)-Pu(IV) solutions gave a quantitative precipitation of both U and Pu. Neither Am(III) nor Np(V) was precipitated in the selective U precipitation and the simultaneous U-Pu precipitation. These results demonstrate the feasibility of the reprocessing by precipitation with NCP.
solution containing U(VI) and Pu(IV) was achieved by stirring the solution for sufficient time after addition of NCP with ratio of [NCP]/[U]=1.4. Addition of an enough amount of NCP to U(VI)-Pu(VI) or U(VI)-Pu(IV) solutions gave a quantitative precipitation of both U and Pu. Neither Am(III) nor Np(V) was precipitated in the selective U precipitation and the simultaneous U-Pu precipitation. These results demonstrate the feasibility of the reprocessing by precipitation with NCP.
Morita, Yasuji; Kawata, Yoshihisa*; Mineo, Hideaki; Koshino, Nobuyoshi*; Asanuma, Noriko*; Ikeda, Yasuhisa*; Yamasaki, Kazuhiko*; Chikazawa, Takahiro*; Tamaki, Yoshihisa*; Kikuchi, Toshiaki*
Proceedings of International Conference on Nuclear Energy System for Future Generation and Global Sustainability (GLOBAL 2005) (CD-ROM), 6 Pages, 2005/10
N-cyclohexyl-2-pyrrolidone (NCP) can selectively precipitate U(VI) ions in aqueous nitric acid solutions. Utilizing this property, we have been developing a simple reprocessing process of spent nuclear fuel based only on precipitation method. In the first precipitation step, only U is separated by precipitation in a yield of about 70%, and in the second precipitation step both U and Pu are recovered and separated from fission products (FP) and other transuranium elements (TRU). In JAERI, precipitation behaviors of Pu and other TRU were examined experimentally, and the results showed the feasibility of the process establishement.
Yamasaki, Kazuhiko*; Chikazawa, Takahiro*; Tamaki, Yoshihisa*; Kikuchi, Toshiaki*; Morita, Yasuji; Kawata, Yoshihisa*; Mineo, Hideaki; Koshino, Nobuyoshi*; Asanuma, Noriko*; Harada, Masayuki*; et al.
Proceedings of International Conference on Nuclear Energy System for Future Generation and Global Sustainability (GLOBAL 2005) (CD-ROM), 4 Pages, 2005/10
N-cyclohexyl-2-pyrrolidone (NCP), can selectively precipitate U(VI) ions in aqueous nitric acid solutions. Utilizing this property, we have been developing a simple reprocessing process of spent nuclear fuel based only on precipitation method. In the first precipitation step, only U is separated by precipitation in a yield of about 70%, and in the second precipitation step both U and Pu are recovered and separated from fission products (FP) and other transuranium elements (TRU). In the present study, a precipitator and a precipitate separator were designed and built up, and were tested with aspets of operationability and system performance.
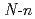 -butyl-2-pyrrolidone,
-butyl-2-pyrrolidone,  -cyclohexylmethyl-2-pyrrolidone, and 1,3-dimethyl-2-imidazolidone
-cyclohexylmethyl-2-pyrrolidone, and 1,3-dimethyl-2-imidazolidoneKoshino, Nobuyoshi*; Harada, Masayuki*; Nogami, Masanobu*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Inorganica Chimica Acta, 358(6), p.1857 - 1864, 2005/03
Times Cited Count:54 Percentile:87.98(Chemistry, Inorganic & Nuclear)Structural analyses of UO (NO
(NO )
) L
L [L=
[L=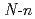 -butyl-2-pyrrolidone, N-cyclohexylmethyl-2-pyrrolidone, and 1,3-dimethyl-2-imidazolidone] have been carried out using X-ray diffraction method. These uranyl complexes were found to have a hexagonal bipyramidal structure. The bond distances of U=O and U-O (ligand), and bond angles of U-O-C(carbonyl) are determined. In uranyl nitrate complexes with cyclic amides such as 2-pyrrolidone, urea, and caprolactam derivatives, a linear correlation was found to hold between U-O (ligand) bond distances and U-O-C(carbonyl) bond angles. Vibrational frequencies of UO
-butyl-2-pyrrolidone, N-cyclohexylmethyl-2-pyrrolidone, and 1,3-dimethyl-2-imidazolidone] have been carried out using X-ray diffraction method. These uranyl complexes were found to have a hexagonal bipyramidal structure. The bond distances of U=O and U-O (ligand), and bond angles of U-O-C(carbonyl) are determined. In uranyl nitrate complexes with cyclic amides such as 2-pyrrolidone, urea, and caprolactam derivatives, a linear correlation was found to hold between U-O (ligand) bond distances and U-O-C(carbonyl) bond angles. Vibrational frequencies of UO (NO
(NO )
) L
L have also been measured by IR and Raman spectrophotometers. Using relationships between vibrational frequencies of O=U=O bonds and donor numbers (DNs) of ligands, donicities of N-substituted-2-pyrrolidones were determined.
have also been measured by IR and Raman spectrophotometers. Using relationships between vibrational frequencies of O=U=O bonds and donor numbers (DNs) of ligands, donicities of N-substituted-2-pyrrolidones were determined.
Koshino, Nobuyoshi*; Harada, Masayuki*; Morita, Yasuji; Kikuchi, Toshiaki*; Ikeda, Yasuhisa*
Progress in Nuclear Energy, 47(1-4), p.406 - 413, 2005/00
Times Cited Count:24 Percentile:81.24(Nuclear Science & Technology)We have developed a simple reprocessing process for spent FBR fuels using N-cyclohexyl-2-pyrrolidone (NCP) which has selective precipitation ability for UO
 ions. It was confirmed that NCP has sufficient precipitation ability for UO
ions. It was confirmed that NCP has sufficient precipitation ability for UO
 ions, decontamination capability (separation of UO
ions, decontamination capability (separation of UO
 from simulated fission products), and resistance to
from simulated fission products), and resistance to  -ray radiation in nitric acid solutions. These findings indicate that NCP is applicable to our reprocessing process. We have also evaluated performances of other precipitants such as N-n-propyl-2-pyrrolidone (NProP), N-n-butyl-2-pyrrolidone (NBP), and N-n-butyl-2-pyridone (NBPyr). It was found that higher decontamination factors (DFs) are obtained by using NProP and NBP. This can be interpreted that the hydrophobicity of NProP and NBP is lower than that of NCP. Furthermore, we have obtained an experimental result that the resistance of NBPyr to
-ray radiation in nitric acid solutions. These findings indicate that NCP is applicable to our reprocessing process. We have also evaluated performances of other precipitants such as N-n-propyl-2-pyrrolidone (NProP), N-n-butyl-2-pyrrolidone (NBP), and N-n-butyl-2-pyridone (NBPyr). It was found that higher decontamination factors (DFs) are obtained by using NProP and NBP. This can be interpreted that the hydrophobicity of NProP and NBP is lower than that of NCP. Furthermore, we have obtained an experimental result that the resistance of NBPyr to  -ray radiation is superior to that of NCP.
-ray radiation is superior to that of NCP.
Kikuchi, Toshiaki*; Yamasaki, Kazuhiko*; Kusama, Makoto*; Chikazawa, Takahiro*; Tamaki, Yoshihisa*; Hanzawa, Masatoshi*; Koshino, Nobuyoshi*; Asanuma, Noriko*; Harada, Masayuki*; Kawata, Yoshihisa*; et al.
no journal, ,
no abstracts in English