Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
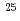 F from
F from 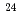 O
O ?
?Tang, T. L.*; Uesaka, Tomohiro*; Kawase, Shoichiro; Beaumel, D.*; Dozono, Masanori*; Fujii, Toshihiko*; Fukuda, Naoki*; Fukunaga, Taku*; Galindo-Uribarri, A.*; Hwang, S. H.*; et al.
Physical Review Letters, 124(21), p.212502_1 - 212502_6, 2020/05
Times Cited Count:19 Percentile:72.96(Physics, Multidisciplinary)The structure of a neutron-rich 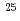 F nucleus is investigated by a quasifree (
F nucleus is investigated by a quasifree (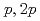 ) knockout reaction. The sum of spectroscopic factors of
) knockout reaction. The sum of spectroscopic factors of 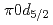 orbital is found to be 1.0
orbital is found to be 1.0  0.3. The result shows that the
0.3. The result shows that the 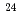 O core of
O core of 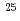 F nucleus significantly differs from a free
F nucleus significantly differs from a free 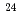 O nucleus, and the core consists of
O nucleus, and the core consists of  35%
35% 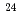 O
O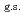 , and
, and  65% excited
65% excited 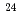 O. The result shows that the
O. The result shows that the 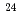 O core of
O core of 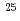 F nucleus significantly differs from a free
F nucleus significantly differs from a free 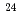 O nucleus. The result may infer that the addition of the
O nucleus. The result may infer that the addition of the 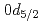 proton considerably changes the neutron structure in
proton considerably changes the neutron structure in 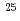 F from that in
F from that in 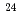 O, which could be a possible mechanism responsible for the oxygen dripline anomaly.
O, which could be a possible mechanism responsible for the oxygen dripline anomaly.
Yonezawa, Yasushige*; Tanaka, Shimpei*; Kubota, Tomomi*; Wakabayashi, Katsuzo*; Yutani, Katsuhide*; Fujiwara, Satoru
Journal of Molecular Biology, 323(2), p.237 - 251, 2002/10
Times Cited Count:76 Percentile:74.42(Biochemistry & Molecular Biology)It is known that hen egg white lysozyme (HEWL) forms amyloid fibrils in highly concentrated ethanol solutions. In order to gain an insight into the mechanism of the amyloid fibril formation, the structures of HEWL in solutions of various protein and ethanol concentrations were investigated with small-angle X-ray and neutron scattering. It was shown that the structural states of HEWL were distinguished as the monomer state, the state of the dimer formation, the state of the protofilament formation, the protofilament state, and the state towards the formation of the amyloid fibrils. Circular dichroism measurements showed that the large changes in the secondary structures of HEWL occurred during the dimer formation. Structural characterization showed that the dimers had an elongated shape, the protofilaments were formed by stacking of the dimers with their long axis (nearly) perpendicular to the protofilament axis, and the changes of the structural states towards the amyloid fibril formation occurred via lateral association of the protofilaments.