Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Ca cast doubt on a doubly magic
Ca cast doubt on a doubly magic  Ca
CaChen, S.*; Browne, F.*; Doornenbal, P.*; Lee, J.*; Obertelli, A.*; Tsunoda, Yusuke*; Otsuka, Takaharu*; Chazono, Yoshiki*; Hagen, G.*; Holt, J. D.*; et al.
Physics Letters B, 843, p.138025_1 - 138025_7, 2023/08
Times Cited Count:1 Percentile:0.02(Astronomy & Astrophysics)Gamma decays were observed in  Ca and
Ca and  Ca following quasi-free one-proton knockout reactions from
Ca following quasi-free one-proton knockout reactions from  Sc. For
Sc. For  Ca, a
Ca, a  ray transition was measured to be 1456(12) keV, while for
ray transition was measured to be 1456(12) keV, while for  Ca an indication for a transition was observed at 1115(34) keV. Both transitions were tentatively assigned as the
Ca an indication for a transition was observed at 1115(34) keV. Both transitions were tentatively assigned as the 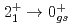 decays. A shell-model calculation in a wide model space with a marginally modified effective nucleon-nucleon interaction depicts excellent agreement with experiment for
decays. A shell-model calculation in a wide model space with a marginally modified effective nucleon-nucleon interaction depicts excellent agreement with experiment for 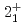 level energies, two-neutron separation energies, and reaction cross sections, corroborating the formation of a new nuclear shell above the N = 34 shell. Its constituents, the
level energies, two-neutron separation energies, and reaction cross sections, corroborating the formation of a new nuclear shell above the N = 34 shell. Its constituents, the 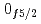 and
and 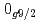 orbitals, are almost degenerate. This degeneracy precludes the possibility for a doubly magic
orbitals, are almost degenerate. This degeneracy precludes the possibility for a doubly magic  Ca and potentially drives the dripline of Ca isotopes to
Ca and potentially drives the dripline of Ca isotopes to 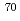 Ca or even beyond.
Ca or even beyond.
 Sb
Sb O
O F
F observed by high-resolution synchrotron and neutron diffraction
observed by high-resolution synchrotron and neutron diffractionShimono, Seiya*; Ishibashi, Hiroki*; Nagayoshi, Yusuke*; Ikeno, Hidekazu*; Kawaguchi, Shogo*; Hagihara, Masato; Torii, Shuki*; Kamiyama, Takashi*; Ichihashi, Katsuya*; Nishihara, Sadafumi*; et al.
Journal of Physics and Chemistry of Solids, 163, p.110568_1 - 110568_7, 2022/04
Times Cited Count:1 Percentile:15.7(Chemistry, Multidisciplinary) VAl/Fe layered films showing exchange bias
VAl/Fe layered films showing exchange biasKubota, Takahide*; Shimada, Yusuke*; Tsuchiya, Tomoki*; Yoshikawa, Tomoki*; Ito, Keita*; Takeda, Yukiharu; Saito, Yuji; Konno, Toyohiko*; Kimura, Akio*; Takanashi, Koki*
Nanomaterials (Internet), 11(7), p.1723_1 - 1723_11, 2021/07
Times Cited Count:2 Percentile:17.84(Chemistry, Multidisciplinary)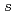 -Orbital component in the Borromean nucleus
-Orbital component in the Borromean nucleus  B
BYang, Z. H.*; Kubota, Yuki*; Corsi, A.*; Yoshida, Kazuki; Sun, X.-X.*; Li, J. G.*; Kimura, Masaaki*; Michel, N.*; Ogata, Kazuyuki*; Yuan, C. X.*; et al.
Physical Review Letters, 126(8), p.082501_1 - 082501_8, 2021/02
Times Cited Count:43 Percentile:96.7(Physics, Multidisciplinary)A quasifree ( ,
,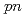 ) experiment was performed to study the structure of the Borromean nucleus
) experiment was performed to study the structure of the Borromean nucleus  B, which had long been considered to have a neutron halo. By analyzing the momentum distributions and exclusive cross sections, we obtained the spectroscopic factors for
B, which had long been considered to have a neutron halo. By analyzing the momentum distributions and exclusive cross sections, we obtained the spectroscopic factors for 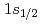 and
and 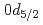 orbitals, and a surprisingly small percentage of 9(2)% was determined for
orbitals, and a surprisingly small percentage of 9(2)% was determined for 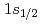 . Our finding of such a small
. Our finding of such a small 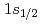 component and the halo features reported in prior experiments can be explained by the deformed relativistic Hartree-Bogoliubov theory in continuum, revealing a definite but not dominant neutron halo in
component and the halo features reported in prior experiments can be explained by the deformed relativistic Hartree-Bogoliubov theory in continuum, revealing a definite but not dominant neutron halo in  B. The present work gives the smallest
B. The present work gives the smallest 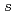 - or
- or  -orbital component among known nuclei exhibiting halo features and implies that the dominant occupation of
-orbital component among known nuclei exhibiting halo features and implies that the dominant occupation of 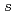 or
or  orbitals is not a prerequisite for the occurrence of a neutron halo.
orbitals is not a prerequisite for the occurrence of a neutron halo.
Kurihara, Momo*; Yasutaka, Tetsuo*; Aono, Tatsuo*; Ashikawa, Nobuo*; Ebina, Hiroyuki*; Iijima, Takeshi*; Ishimaru, Kei*; Kanai, Ramon*; Karube, Jinichi*; Konnai, Yae*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 322(2), p.477 - 485, 2019/11
Times Cited Count:2 Percentile:21.58(Chemistry, Analytical)We assessed the repeatability and reproducibility of methods for determining low dissolved radiocesium concentrations in freshwater in Fukushima. Twenty-one laboratories pre-concentrated three of 10 L samples by five different pre-concentration methods (prussian-blue-impregnated filter cartridges, coprecipitation with ammonium phosphomolybdate, evaporation, solid-phase extraction disks, and ion-exchange resin columns), and activity of radiocesium was measured. The z-scores for all of the  Cs results were within
Cs results were within  2, indicating that the methods were accurate. The relative standard deviations (RSDs) indicating the variability in the results from different laboratories were larger than the RSDs indicating the variability in the results from each separate laboratory.
2, indicating that the methods were accurate. The relative standard deviations (RSDs) indicating the variability in the results from different laboratories were larger than the RSDs indicating the variability in the results from each separate laboratory.
Watanabe, Yusuke; Hayashida, Kazuki; Kato, Toshihiro; Kubota, Mitsuru; Aosai, Daisuke*; Kumamoto, Yoshiharu*; Iwatsuki, Teruki
JAEA-Data/Code 2018-002, 108 Pages, 2018/03
Japan Atomic Energy Agency has been investigating groundwater chemistry to understand the effect of excavation and maintenance of underground facilities as part of the Mizunami Underground Research Laboratory (MIU) Project in Mizunami, Gifu, Japan. In this report, we compiled data of groundwater chemistry and microbiology obtained at the MIU in the fiscal year 2016 and 2014 to 2016, respectively. In terms of ensuring traceability of data, basic information (e.g. sampling location, sampling time, sampling method and analytical method) and methodology for quality control are described.
Kubota, Fukiko*; Shimobori, Yosuke*; Baba, Yuzo*; Koyanagi, Yusuke*; Shimojo, Kojiro; Kamiya, Noriho*; Goto, Masahiro*
Journal of Chemical Engineering of Japan, 44(5), p.307 - 312, 2011/05
Times Cited Count:57 Percentile:82.68(Engineering, Chemical)The application of ionic liquids as alternatives to conventional organic solvents in extraction processes has been actively investigated. A crucial step towards the practical use of ionic liquids is the development of extractants that work effectively within these new media. In the present study, the extraction separation of rare earth metals into ionic liquids, 1-butyl, 1-octyl, and l-dodecyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C min][Tf
min][Tf N],
N],  = 4, 8, 12), was performed using a novel extractant,
= 4, 8, 12), was performed using a novel extractant,  ,
, -dioctyldiglycol amic acid (DODGAA). Quantitative extraction of metal ions such as Y
-dioctyldiglycol amic acid (DODGAA). Quantitative extraction of metal ions such as Y and Eu
and Eu was selectively achieved in the presence of the base metal ion Zn
was selectively achieved in the presence of the base metal ion Zn , which was not extracted at all under the present experimental conditions. The extraction efficiency was enhanced for the shorter-alkyl-chain imidazolium ionic liquid [C
, which was not extracted at all under the present experimental conditions. The extraction efficiency was enhanced for the shorter-alkyl-chain imidazolium ionic liquid [C mim] [Tf
mim] [Tf N] compared to that for a conventional organic solvent system. Extraction mechanism studies elucidated that the metal extraction proceeds via proton exchange reactions between DODGAA and the metal ions in the ionic liquid (the same mechanism as in the conventional organic solvent). The stripping reaction, or recovery, of the metal ions from the extracting phase was readily accomplished with an acid solution such as nitric acid.
N] compared to that for a conventional organic solvent system. Extraction mechanism studies elucidated that the metal extraction proceeds via proton exchange reactions between DODGAA and the metal ions in the ionic liquid (the same mechanism as in the conventional organic solvent). The stripping reaction, or recovery, of the metal ions from the extracting phase was readily accomplished with an acid solution such as nitric acid.
Kubota, Fukiko*; Shimobori, Yosuke*; Koyanagi, Yusuke*; Shimojo, Kojiro; Kamiya, Noriho*; Goto, Masahiro*
Analytical Sciences, 26(3), p.289 - 290, 2010/03
Times Cited Count:49 Percentile:82.68(Chemistry, Analytical)We have developed a highly stable supported liquid membrane based on ionic liquids (ILs) for the separation of rare-earth metals, employing  -dioctyldiglycol amic acid as a mobile carrier. The quantitative transport of Y and Eu through the membrane was successfully attained, and separation from metal impurities, Zn, was efficiently accomplished. A membrane stable enough for long-term operation was constructible from imidazolium-based ILs having a longer alkyl chain, such as octyl or dodecyl groups in an imidazolium cation.
-dioctyldiglycol amic acid as a mobile carrier. The quantitative transport of Y and Eu through the membrane was successfully attained, and separation from metal impurities, Zn, was efficiently accomplished. A membrane stable enough for long-term operation was constructible from imidazolium-based ILs having a longer alkyl chain, such as octyl or dodecyl groups in an imidazolium cation.
Kubota, Fukiko*; Shimobori, Yosuke*; Koyanagi, Yusuke*; Nakashima, Kazunori*; Shimojo, Kojiro; Kamiya, Noriho*; Goto, Masahiro*
Solvent Extraction Research and Development, Japan, 16, p.142 - 146, 2009/00
Kubota, Fukiko*; Koyanagi, Yusuke*; Nakashima, Kazunori*; Shimojo, Kojiro; Kamiya, Noriho*; Goto, Masahiro*
Solvent Extraction Research and Development, Japan, 15, p.81 - 87, 2008/00
Extraction of lanthanide ions was carried out with the organophosphorous extractant PC-88A dissolved in an imidazolium-based ionic liquid. PC-88A was soluble in ionic liquids having long-chain alkyl substituents such as octyl and dodecyl groups, [C mim] and [C
mim] and [C mim][TFSI], although short-chain imidazolium ionic liquids could not solubilize the extractant. The extraction equilibrium observed in the ionic liquid system was similar to that in the n-dodecane system, and the extraction behavior could be explained by the same proton exchange mechanism as for the conventional organic solvent system, although the extraction efficiency was not improved. An ionic liquid having shorter alkyl chain [C
mim][TFSI], although short-chain imidazolium ionic liquids could not solubilize the extractant. The extraction equilibrium observed in the ionic liquid system was similar to that in the n-dodecane system, and the extraction behavior could be explained by the same proton exchange mechanism as for the conventional organic solvent system, although the extraction efficiency was not improved. An ionic liquid having shorter alkyl chain [C mim] showed a higher extraction ability than [C
mim] showed a higher extraction ability than [C mim][TFSI]. Stripping of the metal ions from the ionic liquids was also examined. Quantitative stripping of the metal ions was achieved by nitric acid in the [C
mim][TFSI]. Stripping of the metal ions from the ionic liquids was also examined. Quantitative stripping of the metal ions was achieved by nitric acid in the [C mim][TFSI] system.
mim][TFSI] system.
Kubota, Fukiko*; Koyanagi, Yusuke*; Nakashima, Kazunori*; Shimojo, Kojiro; Kamiya, Noriho*; Goto, Masahiro*
Solvent Extraction Research and Development, Japan, 14, p.115 - 120, 2007/00
We carried out the extraction of a protein cytochrome c (Cyt-c) with an ionic liquid with an attached crown ether moiety in the imidazolium cation. The functional ionic liquid, [18C6mim][PF ], was dissolved in two different ionic liquids as diluents, one having a hydroxyethyl group, [C
], was dissolved in two different ionic liquids as diluents, one having a hydroxyethyl group, [C OHmim][Tf
OHmim][Tf N], and the other an ethyl group, [C
N], and the other an ethyl group, [C mim][Tf
mim][Tf N], respectively. The transfer of Cyt-c from the aqueous phase to the ionic liquid phase took place with [18C6mim][PF
N], respectively. The transfer of Cyt-c from the aqueous phase to the ionic liquid phase took place with [18C6mim][PF ] in the [C
] in the [C OHmim][Tf
OHmim][Tf N] system. Although the extraction ability was lower than that of dicyclohexano-18-crown-6 (DCH18C6), the functionalized ionic liquid system gave a better stripping performance than the ordinary crown ether system. The extraction efficiency was not influenced by operating temperature when the concentration of [18C6mim] was high whereas it was greatly affected by temperature at low [18C6mim] concentration.
N] system. Although the extraction ability was lower than that of dicyclohexano-18-crown-6 (DCH18C6), the functionalized ionic liquid system gave a better stripping performance than the ordinary crown ether system. The extraction efficiency was not influenced by operating temperature when the concentration of [18C6mim] was high whereas it was greatly affected by temperature at low [18C6mim] concentration.
Tanabe, Tetsuro*; Fujine, Michihiko*; Noguchi, Hiroshi*; Yagi, Yasufumi*; Hirano, Yoichi*; Shimizu, Hajime*; Akiba, Masato; Araki, Masanori; Kubota, Yusuke*; Miyahara, Akira*
Journal of Nuclear Materials, 200(1), p.120 - 127, 1993/03
Times Cited Count:9 Percentile:66.93(Materials Science, Multidisciplinary)no abstracts in English
Kubota, Fukiko*; Koyanagi, Yusuke*; Nakashima, Kazunori*; Shimojo, Kojiro; Kamiya, Noriho*; Goto, Masahiro*
no journal, ,
no abstracts in English
Kubota, Fukiko*; Koyanagi, Yusuke*; Nakashima, Kazunori*; Shimojo, Kojiro; Kamiya, Noriho*; Goto, Masahiro*
no journal, ,
no abstracts in English
Ozawa, Yusuke*; Kubota, Hitoshi*; Miura, Kenta*; Hanaizumi, Osamu*; Noguchi, Katsuya*; Sato, Takahiro; Ishii, Yasuyuki; Koka, Masashi; Takano, Katsuyoshi*; Okubo, Takeru; et al.
no journal, ,
Miura, Kenta*; Sato, Takahiro; Ishii, Yasuyuki; Koka, Masashi; Takano, Katsuyoshi*; Okubo, Takeru; Yamazaki, Akiyoshi; Kada, Wataru; Yokoyama, Akihito; Kamiya, Tomihiro; et al.
no journal, ,
Miura, Kenta*; Hanaizumi, Osamu*; Kada, Wataru*; Ozawa, Yusuke*; Inada, Kazuki*; Kubota, Atsushi*; Kawashima, Akihiro*; Sato, Takahiro; Ishii, Yasuyuki; Koka, Masashi; et al.
no journal, ,
no abstracts in English
Nobuhara, Fumiyoshi*; Matsuda, Norihiro; Onishi, Seiki*; Matsui, Yusuke*; Kubota, Osamu*; Sakamoto, Yukio*; Hirao, Yoshihiro*
no journal, ,
no abstracts in English