Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Wei, Y.*; Hoshi, Harutaka; Kumagai, Mikio*
Proceedings of 16th Pacific Basin Nuclear Conference (PBNC-16) (CD-ROM), 6 Pages, 2008/10
To separate the long-lived minor actinides (MA=Am, Cm) from high level liquid waste, we have been studying an advanced separation process by extraction chromatography. The process consists of two separation columns packed with CMPO (octyl(phenyl)-N,N-diisobutylcarbamoyl-methyl phosphine oxide) extraction resin for elemental group separation and a soft-donor named R-BTP (2,6-bis-(5,6-dialkyl-1,2,4-triazine-3-yl)pyridine) extraction resin for the isolation of MA from lanthanides (Ln), respectively. In this work, a hot test for the separation of MA from MA containing effluent from the irradiated MOX-fuel treatment process was carried out using a column packed with R-BTP extraction resin. It was found that a complete separation between MA and Ln was achieved. In addition, small amounts of U and Pu remained in the MA-Ln effluent could be effectively recovered together with the MA. The test results indicate that the proposed MA separation process is essentially feasible.
Hoshi, Harutaka*; Wei, Y.-Z.*; Kumagai, Mikio*; Asakura, Toshihide; Morita, Yasuji
Journal of Alloys and Compounds, 444-445, p.663 - 667, 2007/10
Times Cited Count:4 Percentile:32.43(Chemistry, Physical)no abstracts in English
Hoshi, Harutaka*; Wei, Y.*; Kumagai, Mikio*; Asakura, Toshihide; Morita, Yasuji
Recent Advances in Actinide Science, p.596 - 598, 2006/06
Recently, extraction selectivity for trivalent minor actinides (MA = Am and Cm) over lanthanides (Ln) has been found in some extractants containing soft donor, such as S or N, ligands. Kolarik et al. reported that a new N-donor ligand 2,6-bis(5,6-dialkyl-1,2,4-triazine-3-yl)-pyridine (R-BTP) shows high selectivity for MA (III) over Ln(III) [1]. However, protonation of R-BTP results in its acidic hydrolysis in acidic medium. Stability in acidic solution was improved by substitution of long normal chain or branched chain [2]. In this work, separation of MA(III) and Ln(III) from nitric acid solution was studied by using novel R-BTP impregnated resin. Branched R-BTP resin had high affinity for Am from up to 4 M HNO solution and its distribution coefficient was over 10
solution and its distribution coefficient was over 10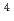 .
.
Hoshi, Harutaka*; Wei, Y.*; Kumagai, Mikio*; Asakura, Toshihide; Morita, Yasuji
Journal of Alloys and Compounds, 408-412, p.1274 - 1277, 2006/02
Times Cited Count:40 Percentile:83.98(Chemistry, Physical)For the development of advanced aqueous reprocessing system, it is one of the most important subjects to separate minor trivalent actinides (MA = Am and Cm). Recently, extraction selectivity for MA(III) over Ln(III) has been found in some extractants containing soft donor, such as S or N, ligands. Kolarik et al. reported that a new N-donor ligand 2,6-bis(5,6-dialkyl-1,2,4-triazine-3-yl)-pyridine (R-BTP) shows high selectivity for MA (III) over Ln(III). The novel silica-based extraction resins were prepared by impregnating some R-BTP molecules into a macroreticular styrene-divinylbenzene copolymer which is immobilized in porous silica particles with a mean diameter of 50  m. Separation of simulated high level liquid waste solution containing Ln(III) and trace amount of Am(III) was studied. Am(III) was mutually separated from Ln(III) through a packed column with R-BTP impregnating resin, very high decontamination factor (
m. Separation of simulated high level liquid waste solution containing Ln(III) and trace amount of Am(III) was studied. Am(III) was mutually separated from Ln(III) through a packed column with R-BTP impregnating resin, very high decontamination factor ( 10
10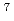 ) for Am, and all the elements were recovered quantitatively.
) for Am, and all the elements were recovered quantitatively.
Hoshi, Harutaka*; Wei, Y.*; Kumagai, Mikio*; Asakura, Toshihide; Morita, Yasuji
Proceedings of International Conference on Nuclear Energy System for Future Generation and Global Sustainability (GLOBAL 2005) (CD-ROM), 6 Pages, 2005/10
For the development of nuclear fuel cycle, it is one of the most important tasks to improve reprocessing more economically and efficiently. Especially, to establish the Fast Breeder Reactor (FBR) cycle system for the future, it is strongly desirable to develop a new reprocessing which uses more compact equipments and produces less radioactive wastes compared to the present PUREX process. For this purpose, we have proposed a novel aqueous reprocessing system named ERIX Process to treat spent FBR-MOX fuels. This process consists of (1) Pd removal by selective adsorption using a specific anion exchanger; (2) electrolytic reduction for the valence adjustment of the major actinides including U, Pu, Np and some fission products (FP) such as Tc and Ru; (3) anion exchange separation for the recovery of U, Pu and Np using a new type of anion exchanger, AR-01; and (4) selective separation of long-lived minor actinides (MA = Am and Cm) by extraction chromatography. In this work, MA separation process was studied.
Hoshi, Harutaka*; Wei, Y.*; Kumagai, Mikio*; Asakura, Toshihide; Morita, Yasuji
Journal of Radioanalytical and Nuclear Chemistry, 262(3), p.601 - 605, 2005/01
Electroreduction of Tc(VII) in nitric acid solution using grassy carbon electrode was studied. The electroreduction was conducted at a constant potential -300 mV (vs. Ag/AgCl) with a potentiostat. It was found that difference of the Tc concentration in the solutions before and after the electrolysis was negligibly small. This means that there were almost no TcO or Tc deposited on the carbon fiber electrode during the electroreduction. Absorption spectra and distribution coefficients obtained by ion-exchange analysis indicated that Tc(VII) was reduced to Tc(IV).
or Tc deposited on the carbon fiber electrode during the electroreduction. Absorption spectra and distribution coefficients obtained by ion-exchange analysis indicated that Tc(VII) was reduced to Tc(IV).
Hoshi, Harutaka*; Wei, Y.*; Kumagai, Mikio*; Asakura, Toshihide; Uchiyama, Gunzo*
Journal of Nuclear Science and Technology, 39(Suppl.3), p.874 - 877, 2002/11
To facilitate the management of high-level liquid waste (HLLW) and minimize its long-term radiological risk in geologic disposal, we have proposed an advanced partitioning process by extraction chromatography using a minimal organic solvent and compact equipment to separate long-lived minor actinides (MA) and specific fission products (FP) such as Zr and Mo from nitrate acidic HLLW solution. Novel silica-based extraction-resin for elemental groups separation was prepared by impregnating CMPO (octyl(phenyl)-N, N-diisobutylcarbamoylmethylphosphine oxide) into a macro-reticular styrene-divinylbenzene copolymer immobilized in porous silica particles with a diameter of 50  m (SiO
m (SiO -P). Separation experiments for simulated HLLW solutions containing a trace amount of
-P). Separation experiments for simulated HLLW solutions containing a trace amount of 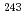 Am (III) and macro amounts of typical FP elements were carried out by column chromatography. It was found that the elements in the simulated HLLW were successfully separated to the following three groups: Cs-Sr-Rh-Ru, Pd-Ln-Am and Zr-Mo.
Am (III) and macro amounts of typical FP elements were carried out by column chromatography. It was found that the elements in the simulated HLLW were successfully separated to the following three groups: Cs-Sr-Rh-Ru, Pd-Ln-Am and Zr-Mo.
Asakura, Toshihide; Uchiyama, Gunzo; Hoshi, Harutaka*; Wei, Y.*; Kumagai, Mikio*
Journal of Nuclear Science and Technology, 39(Suppl.3), p.340 - 342, 2002/11
no abstracts in English
Wei, Y.*; Hoshi, Harutaka*; Kumagai, Mikio*; Asakura, Toshihide; Uchiyama, Gunzo*
Journal of Nuclear Science and Technology, 39(Suppl.3), p.761 - 764, 2002/11
To separate long-lived minor actinides and specific fission products such as Zr and Mo from nitrate acidic high-level liquid waste, we studied an advanced partitioning process by extraction chromatography using minimal organic solvent and compact equipment. In this work, we synthesized several new type of nitrogen donor ligands, 2,6-bi-(5,6-dialkyl-1,2,4-triazine-3-yl)-pyridine (R-BTP) with different alkyl groups and prepared novel silica-based extraction-resins by impregnating these ligands into the SiO -P support with a diameter of 50
-P support with a diameter of 50  m. The adsorption performance of
m. The adsorption performance of 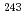 Am and Ln (III) from nitrate solution was investigated. It was found that the adsorption behavior depends strongly on the alkyl group in R-BTP.
Am and Ln (III) from nitrate solution was investigated. It was found that the adsorption behavior depends strongly on the alkyl group in R-BTP.  Bu-BTP/SiO
Bu-BTP/SiO -P and
-P and  Hex-BTP/SiO
Hex-BTP/SiO -P showed high absorbability and selectivity for Am (III) over Ln (III). The separation factor is about 10
-P showed high absorbability and selectivity for Am (III) over Ln (III). The separation factor is about 10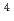 for Am/Ce and near 10
for Am/Ce and near 10 for Am/Eu-Gd, respectively. Effective Am (III) separation form Ln (III) by extraction chromatography using R-BTP/SiO
for Am/Eu-Gd, respectively. Effective Am (III) separation form Ln (III) by extraction chromatography using R-BTP/SiO -P extraction-resins is expected.
-P extraction-resins is expected.
Kuraoka, Etsushu*; ; Kumagai, Mikio*;
JNC TJ8400 2002-005, 58 Pages, 2002/07
In order to study the possibility of a pilot-plant-scale test for MA recovery process by extraction chromatography which has been evaluated to have the potentials of small construction area and low cost, this work investigated the process flow sheet, the configuration and installation layout of equipment. Furthermore, the treatment methods for the organic wastes such as CMPO adsorbent were examined. The proposed process consists of two separation columns packed with CMPO adsorbent, where the 1st one is for the recovery of MA, heavy RE, Zr and Mo from the HLLW and the 2nd one for the removal of the Zr and Mo from the effluent of the 1st column, recovering the MA and the heavy RE as a nitrate acidic solution. The results of the separation experiments using simulated HLLW solutions indicate that the expected separation performance for the main elements was achieved and the proposed process is principally applicable. In addition, the process has the advantages such as "salt-free", the possible recovery of Zr-Mo and Pd from HLLW. The process flow diagram for the proposed separation process was drawn and the mass balance for one test of pilot-plant-scale was evaluated. The structure and size of the main equipment including the separation columns were conceptually designed and the installation layout of the equipment was drawn. The cost of the equipment was preliminary estimated based the specification of the equipment. In addition, the effective treatment methods for the organic wastes including depleted CMPO adsorbent, DTPA or oxalic acid containing waste solution were examined based on article survey. Moreover, some exploratory experiments for the decomposition treatment of these wastes were conducted by using the Fenton reagents. Finally, the subjects remained in this process were extracted.
; Arai, Tsuyoshi*; Kumagai, Mikio*
JNC TJ9400 2001-012, 92 Pages, 2001/03
In order to develop an economically efficient wet separation process other than solvent extraction for reprocessing spent FBR-fuel (MOX fuel), we have investigated the possibility of an advanced ion exchange process. Based on the results of fundamental research and the fruits of this research in last year, the proposed FBR-fuel reprocessing process which consists of anion exchange separation and extraction chromatography separation has been studicd quantitatively from the engineering aspect. The plant concept, construction cost, applicability of this process were investigated and preliminarily evaluated. The proposed process was improved to reduce the amounts of operation solution and waste generation, and to enhance the properties of the impregnation adsorbents for MA separation. The mass balance including waste generation in main processes was evaluated. The operation flow sheets for each process were drawn. The main machines were conceptually designed. Furthermore, conceptual design for the reprocessing plant using ion exchange and extraction chromatography was executed and the installation layouts of the machines, equipment and facilities were examined and designed. Based on the research results, the construction cost for the reprocessing plant was estimated and compared with the existing PUREX plant. Finally, the subjects resulted from the introduction of the ion exchange process were extracted and the considerations for solving these subjects were also indicated.
; Arai, Tsuyoshi*; Kumagai, Mikio*
JNC TJ9400 2000-002, 80 Pages, 2000/02
In order to develop an economically efficient wet separation process other than solvent extraction for reprocessing spent FBR-fuel (MOX fuel), we have investigated the possibility of an advanced ion exchange process. Based on the fundamental research results, we proposed an advanced ion exchange process considering the characteristics of FBR-fuel cycle. The separation system consists of a main separation process using a novel anion exchanger which has a rapid kinetics and two extraction chromatography processes for minor actinides isolation using novel impregnation adsorbents with high selectivity. The chemical flow sheet, mass balance chart, list of main equipment and installation layout of each equipment were estimated and designed for the process in a reprocessing plant with the capacity of 200 tHM/y FBR-fuel. The process was pfeliminarny evalualed from the aspects of economy performance, recovery of potentially useable resources, minimization of environmental risk and proliferation-resistance by comparing with the advanced PUREX process. Furthermore, the subjects which are important for the practical application of the process are also listed.
Sazarashi, Masami*; ; Ikeda, Yasuhisa*; Kumagai, Mikio*; ;
PNC TJ1564 97-002, 20 Pages, 1997/03
None
Inami, Shinichi; Nogami, Takashi; Maki, Akira; Ogata, Yoshiaki; Takeshita, Kenji*; Sazarashi Masami*; Kumagai, Mikio*
Donen Giho, (98), p.32 - 42, 1996/06
None
Sazarashi, Masami*; Ikeda, Yasuhisa*; Kumagai, Mikio*; ;
PNC TJ1564 96-002, 19 Pages, 1996/03
None
Sazarashi, Masami*; Ikeda, Yasuhisa*; Kumagai, Mikio*
PNC TJ1564 93-003, 17 Pages, 1993/02
The present study has been carrisd out to establish the final disposal methods for e wastes containing radioactive iodines. In the previous studies, cinnabar (spain) a montmorillonite containing Ag-thiourea complex were selected as the arificial barri materials. In the present report, the adsorption mechanisms of two adsorbents were udied in more detail. Furthermore, various synthetic inorganic ion exchangers were ao examined as the model materials of I ion adsorbents. The results are summarized afollow : 1. Detail examination for I
ion adsorbents. The results are summarized afollow : 1. Detail examination for I ion adsorption behavior of cinnabar (1) In avaible cinnabars, only spain one has an adsorptivity to I
ion adsorption behavior of cinnabar (1) In avaible cinnabars, only spain one has an adsorptivity to I ion. (2) The cinnabar adsorde has the selective adsorptivity to I
ion. (2) The cinnabar adsorde has the selective adsorptivity to I ion. (3) It takes 40 days to reach the adsorpti equilibrium. (4) The amount of adsorbed I
ion. (3) It takes 40 days to reach the adsorpti equilibrium. (4) The amount of adsorbed I ions decreases with the rise of pH, and t desorption of I
ions decreases with the rise of pH, and t desorption of I ions from cinnadar is not observed. These results suggest that thedsorption of I
ions from cinnadar is not observed. These results suggest that thedsorption of I ions on cinnabar is not due to the ion exchange but the compo
ions on cinnabar is not due to the ion exchange but the compo
Sazarashi, Masami*; Ikeda, Yasuhisa*; Kumagai, Mikio*
PNC TJ1564 93-002, 23 Pages, 1993/02
None
; Ikeda, Yasuhisa*; Kumagai, Mikio*
PNC TJ1262 92-003, 24 Pages, 1992/02
THE PRESENT STUDY HAVE BEEN CARRIED OUT TO ESTABLISH THE FINAL DISPOSAL METHODS FORTHE WASTES CONTAINING RADIOACTIVE IODINES. FROM THE PREVIOUS STUDIES,IT WAS CLARIFIEDTHAT THE IODINE SPECIES EXIST AS I-IONS IN THE GROUND WATER AND THAT ANIONS SUCH AS I-IONS ARE LITTLE ADSORBED ON THE NATURAL MINERALS. HENCE,THREE KINDS OF ADSORBENTS,I.E.,CINNABAR,MONTMORILLONITES CONTAINING AG-THIOUREA COMPLEX,AND INORGANIC ION EXCHANGERS,WERE EXAMINED AS THE ARTIFICIAL BARRIER MATERIALS. FURTHERMORE,THE HYDROHORBIC IODINE ADSORBENTS WERE STUDIED FROM THE VIEWPOINT OF LEACHING-RESISTANT. THE RESULTS ARE SUMMARIZED AS FOLLOWS:1.ADSORPTION MECHANISM OF CINNABAR1.THE ADSORPTION OF I-IONS ON THE CINNABAR WAS NOT AFFECTED BY AN INCREASE INTEMPERATURE AND THE COEXISTENT IONS. THESE RESULTS SUGGEST THAT THE ADSORPTION OF I-IONS ON CINNABAR TAKES PLACE THROUGH MECHANISM EXCEPT FOR THE ION EXCHANGE.2.THE AMOUNT OF I-IONS ADSORBED ON THE CINNABAR WAS FOUND TO BE AROUND 10-5MOL/G,WHICH IS EXTREMELY SMALLER THAN