Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...

McGrady, J.; Kumagai, Yuta; Watanabe, Masayuki; Kirishima, Akira*; Akiyama, Daisuke*; Kimuro, Shingo; Ishidera, Takamitsu
Journal of Nuclear Science and Technology, 60(12), p.1586 - 1594, 2023/12
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)Sato, Nobuaki*; Kirishima, Akira*; Sasaki, Takayuki*; Takano, Masahide; Kumagai, Yuta; Sato, Soichi; Tanaka, Kosuke
Current Location of Fuel Debris Chemistry, 178 Pages, 2023/11
Considerable efforts have been devoted to the decommissioning of the TEPCO's Fukushima Daiichi Nuclear Power Station (1F) and now the retrieval of fuel debris is being proceeded on a trial basis. It can be said that the succession of science and technology related to debris, that is, human resource development, is important and indispensable. For that reason, we thought that a specific textbook on decommissioning is necessary. Regarding the 1F fuel debris, we still do not know enough, and it would be difficult to describe the details. However, 12 years have passed since the accident, and we have come to understand the situation of 1F to a certain extent. At this stage, it is essential for future development to organize the current situation by combining examples of past severe accidents. Therefore, we presented in this book the current state of fuel debris chemistry research from the perspectives of solid chemistry, solution chemistry, analytical chemistry, radiochemistry, and radiation chemistry.
Kusaka, Ryoji; Kumagai, Yuta; Watanabe, Masayuki; Sasaki, Takayuki*; Akiyama, Daisuke*; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Science and Technology, 60(5), p.603 - 613, 2023/05
Times Cited Count:1 Percentile:31.61(Nuclear Science & Technology)Kumagai, Yuta
Hoshasen Kagaku (Internet), (115), p.43 - 49, 2023/04
Oxidation and dissolution of uranium oxide materials has been a subject of numerous studies as a basis of the geological disposal technology for spent nuclear fuel. The understandings obtained by these studies provide useful suggestions for research and development regarding the retrieval and storage of nuclear fuel debris generated by a nuclear severe accident. Here, these research backgrounds of oxidative dissolution of uranium oxides are briefly reviewed and some studies relating to radiation-induced reactions will be introduced.
 , Zr, and stainless steel and leaching behavior of the fission products and matrix elements
, Zr, and stainless steel and leaching behavior of the fission products and matrix elementsTonna, Ryutaro*; Sasaki, Takayuki*; Kodama, Yuji*; Kobayashi, Taishi*; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Kumagai, Yuta; Kusaka, Ryoji; Watanabe, Masayuki
Nuclear Engineering and Technology, 55(4), p.1300 - 1309, 2023/04
Times Cited Count:1 Percentile:72.91(Nuclear Science & Technology)Simulated debris was synthesized using UO , Zr, and stainless steel and a heat treatment method under inert or oxidizing conditions. The primary U solid phase of the debris synthesized at 1473 K under inert conditions was UO
, Zr, and stainless steel and a heat treatment method under inert or oxidizing conditions. The primary U solid phase of the debris synthesized at 1473 K under inert conditions was UO , whereas a (U,Zr)O
, whereas a (U,Zr)O solid solution formed at 1873 K. Under oxidizing conditions, a mixture of U
solid solution formed at 1873 K. Under oxidizing conditions, a mixture of U O
O and (Fe,Cr)UO
and (Fe,Cr)UO phases formed at 1473 K whereas a (U,Zr)O
phases formed at 1473 K whereas a (U,Zr)O solid solution formed at 1873 K. The leaching behavior of the fission products from the simulated debris was evaluated using two methods: the irradiation method, for which fission products were produced via neutron irradiation, and the doping method, for which trace amounts of non-radioactive elements were doped into the debris. The dissolution behavior of U depended on the properties of the debris and aqueous medium the debris was immersed in. Cs, Sr, and Ba leached out regardless of the primary solid phases. The leaching of high-valence Eu and Ru ions was suppressed, possibly owing to their solid-solution reaction with or incorporation into the uranium compounds of the simulated debris.
solid solution formed at 1873 K. The leaching behavior of the fission products from the simulated debris was evaluated using two methods: the irradiation method, for which fission products were produced via neutron irradiation, and the doping method, for which trace amounts of non-radioactive elements were doped into the debris. The dissolution behavior of U depended on the properties of the debris and aqueous medium the debris was immersed in. Cs, Sr, and Ba leached out regardless of the primary solid phases. The leaching of high-valence Eu and Ru ions was suppressed, possibly owing to their solid-solution reaction with or incorporation into the uranium compounds of the simulated debris.
 -edge to assess the U(V) electronic structure in FeUO
-edge to assess the U(V) electronic structure in FeUO
Yomogida, Takumi; Akiyama, Daisuke*; Ouchi, Kazuki; Kumagai, Yuta; Higashi, Kotaro*; Kitatsuji, Yoshihiro; Kirishima, Akira*; Kawamura, Naomi*; Takahashi, Yoshio*
Inorganic Chemistry, 61(50), p.20206 - 20210, 2022/12
Times Cited Count:2 Percentile:36.89(Chemistry, Inorganic & Nuclear)FeUO was studied to clarify the electronic structure of U(V) in a metal monouranate compound. We obtained the peak splitting of HERFD-XANES spectra utilizing high-energy-resolution fluorescence detection-X-ray absorption near edge structure (HERFD-XANES) spectroscopy at the U L
was studied to clarify the electronic structure of U(V) in a metal monouranate compound. We obtained the peak splitting of HERFD-XANES spectra utilizing high-energy-resolution fluorescence detection-X-ray absorption near edge structure (HERFD-XANES) spectroscopy at the U L -edge, which is a novel technique in the U(V) compounds. Theoretical calculations revealed that the peak splitting was caused by splitting the 6d orbital of U(V). Such distinctive electronic states are of major interest to researchers and engineers working in various fields, from fundamental physics to the nuclear industry and environmental sciences for actinide elements.
-edge, which is a novel technique in the U(V) compounds. Theoretical calculations revealed that the peak splitting was caused by splitting the 6d orbital of U(V). Such distinctive electronic states are of major interest to researchers and engineers working in various fields, from fundamental physics to the nuclear industry and environmental sciences for actinide elements.
 and CrUO
and CrUO
Akiyama, Daisuke*; Kusaka, Ryoji; Kumagai, Yuta; Nakada, Masami; Watanabe, Masayuki; Okamoto, Yoshihiro; Nagai, Takayuki; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Materials, 568, p.153847_1 - 153847_10, 2022/09
Times Cited Count:3 Percentile:68.71(Materials Science, Multidisciplinary)FeUO , CrUO
, CrUO , and Fe
, and Fe Cr
Cr UO
UO are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO
are monouranates containing pentavalent U. Even though these compounds have similar crystal structures, their formation conditions and thermal stability are significantly different. To determine the factors causing the difference in thermal stability between FeUO and CrUO
and CrUO , their crystal structures were evaluated in detail. A Raman band was observed at 700 cm
, their crystal structures were evaluated in detail. A Raman band was observed at 700 cm in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe
in all the samples. This Raman band was derived from the stretching vibration of the O-U-O axis band, indicating that Fe Cr
Cr UO
UO was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M
was composed of a uranyl-like structure in its lattice regardless of its "x"' value. M ssbauer measurements indicated that the Fe in FeUO
ssbauer measurements indicated that the Fe in FeUO and Fe
and Fe Cr
Cr UO
UO were trivalent. Furthermore, Fe
were trivalent. Furthermore, Fe Cr
Cr UO
UO lost its symmetry around Fe
lost its symmetry around Fe with increasing electron densities around Fe
with increasing electron densities around Fe , as the abundance of Cr increased. These results suggested no significant structural differences between FeUO
, as the abundance of Cr increased. These results suggested no significant structural differences between FeUO and CrUO
and CrUO . Thermogravimetric measurements for UO
. Thermogravimetric measurements for UO , FeUO
, FeUO , and CrUO
, and CrUO showed that the temperature at which FeUO
showed that the temperature at which FeUO decomposed under an oxidizing condition (approximately 800
decomposed under an oxidizing condition (approximately 800  C) was significantly lower than the temperature at which the decomposition of CrUO
C) was significantly lower than the temperature at which the decomposition of CrUO started (approximately 1250
started (approximately 1250  C). Based on these results, we concluded that the decomposition of FeUO
C). Based on these results, we concluded that the decomposition of FeUO was triggered by an "in-crystal" redox reaction, i.e., Fe
was triggered by an "in-crystal" redox reaction, i.e., Fe
 U
U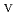
 Fe
Fe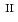
 U
U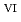 , which would not occur in the CrUO
, which would not occur in the CrUO lattice because Cr
lattice because Cr could never be reduced under the investigated condition. Finally, the existence of Cr
could never be reduced under the investigated condition. Finally, the existence of Cr in FexCr
in FexCr UO
UO effectively suppressed the decomposition of the Fe
effectively suppressed the decomposition of the Fe Cr
Cr UO
UO crystal, even at a very low Cr content.
crystal, even at a very low Cr content.
 , Zr, and stainless-steel
, Zr, and stainless-steelKirishima, Akira*; Akiyama, Daisuke*; Kumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Sasaki, Takayuki*; Sato, Nobuaki*
Journal of Nuclear Materials, 567, p.153842_1 - 153842_15, 2022/08
Times Cited Count:4 Percentile:78.52(Materials Science, Multidisciplinary)To understand the chemical structure and stability of nuclear fuel debris consisting of UO , Zr, and Stainless Steel (SUS) generated by the Fukushima Daiichi Nuclear Power Plant accident in Japan in 2011, simulated debris of the UO
, Zr, and Stainless Steel (SUS) generated by the Fukushima Daiichi Nuclear Power Plant accident in Japan in 2011, simulated debris of the UO -SUS-Zr system and other fundamental component systems were synthesized and characterized. The simulated debris were synthesized by heat treatment for 1 to 12 h at 1600
-SUS-Zr system and other fundamental component systems were synthesized and characterized. The simulated debris were synthesized by heat treatment for 1 to 12 h at 1600 C, in inert (Ar) or oxidative (Ar + 2% O
C, in inert (Ar) or oxidative (Ar + 2% O ) atmospheres.
) atmospheres.  Np and
Np and  Am tracers were doped for the leaching tests of these elements and U from the simulated debris. The characterization of the simulated debris was conducted by XRD, SEM-EDX, Raman spectroscopy, and M
Am tracers were doped for the leaching tests of these elements and U from the simulated debris. The characterization of the simulated debris was conducted by XRD, SEM-EDX, Raman spectroscopy, and M ssbauer spectroscopy, which provided the major uranium phase of the UO
ssbauer spectroscopy, which provided the major uranium phase of the UO  -SUS-Zr debris was the solid solution of U
-SUS-Zr debris was the solid solution of U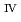 O
O (s.s.) with Zr(IV) and Fe(II) regardless of the treatment atmosphere. The long-term immersion test of the simulated debris in pure water and that in seawater revealed the macro scale crystal structure of the simulated debris was chemically very stable in the wet condition for a year or more. Furthermore, the leaching test results showed that the actinide leaching ratios of U, Np, Am from the UO
(s.s.) with Zr(IV) and Fe(II) regardless of the treatment atmosphere. The long-term immersion test of the simulated debris in pure water and that in seawater revealed the macro scale crystal structure of the simulated debris was chemically very stable in the wet condition for a year or more. Furthermore, the leaching test results showed that the actinide leaching ratios of U, Np, Am from the UO -SUS-Zr debris were very limited and less than 0.08 % for all the experiments in this study.
-SUS-Zr debris were very limited and less than 0.08 % for all the experiments in this study.
 O
O solution
solutionKumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Sasaki, Takayuki*
Journal of Nuclear Science and Technology, 59(8), p.961 - 971, 2022/08
Times Cited Count:2 Percentile:53.91(Nuclear Science & Technology)We investigated potential degradation of fuel debris caused by H O
O , which is the oxidant of major impact from water radiolysis. We performed leaching experiments on different kinds of simulated debris comprising U, Fe, Cr, Ni, and Zr in an aqueous H
, which is the oxidant of major impact from water radiolysis. We performed leaching experiments on different kinds of simulated debris comprising U, Fe, Cr, Ni, and Zr in an aqueous H O
O solution. Chemical analysis of the leaching solution showed that U dissolution was induced by H
solution. Chemical analysis of the leaching solution showed that U dissolution was induced by H O
O . Raman analysis after the leaching revealed that uranyl peroxides were formed on the surface of the simulated debris. These results demonstrate that uranyl peroxides are possible alteration products of fuel debris from H
. Raman analysis after the leaching revealed that uranyl peroxides were formed on the surface of the simulated debris. These results demonstrate that uranyl peroxides are possible alteration products of fuel debris from H O
O reaction. However, the sample in which the main uranium-containing phase was a U-Zr oxide solid solution showed much less uranium dissolution and no Raman signal of uranyl peroxides. Comparison of these results indicates that formation of an oxide solid solution of Zr with UO
reaction. However, the sample in which the main uranium-containing phase was a U-Zr oxide solid solution showed much less uranium dissolution and no Raman signal of uranyl peroxides. Comparison of these results indicates that formation of an oxide solid solution of Zr with UO improves the stability of fuel debris against H
improves the stability of fuel debris against H O
O reaction.
reaction.
 O
O -induced oxidative degradation of simulated fuel debris
-induced oxidative degradation of simulated fuel debrisKumagai, Yuta; Kusaka, Ryoji; Nakada, Masami; Watanabe, Masayuki; Akiyama, Daisuke*; Kirishima, Akira*; Sato, Nobuaki*; Sasaki, Takayuki*
Hoshasen Kagaku (Internet), (113), p.61 - 64, 2022/04
The severe accident at TEPCO's Fukushima Daiichi Nuclear Power Station resulted in generation of fuel debris. The fuel debris is in contact with water and the radiolysis of water can accelerate degradation of the debris. The analysis of particles sampled from inside or near the damaged reactors indicates the complicated compositions of the fuel debris. It is challenging to estimate the effect of water radiolysis on such a complicated material. Therefore, in this study, we investigated the potential degradation process by leaching experiments of simulated fuel debris in aqueous H O
O solution. The results show that the reaction of H
solution. The results show that the reaction of H O
O induced uranium dissolution from most of the samples and then formation of uranyl peroxides. In contrast, a sample that had U-Zr oxide solid solution as the major phase exhibited remarkable resistance to H
induced uranium dissolution from most of the samples and then formation of uranyl peroxides. In contrast, a sample that had U-Zr oxide solid solution as the major phase exhibited remarkable resistance to H O
O . These findings revealed that the degradation of the simulated debris reflects the reactivity and stability of the uranium phase in the matrices.
. These findings revealed that the degradation of the simulated debris reflects the reactivity and stability of the uranium phase in the matrices.
Toigawa, Tomohiro; Kumagai, Yuta; Yamashita, Shinichi*; Ban, Yasutoshi; Matsumura, Tatsuro
UTNL-R-0502 (Internet), 2 Pages, 2022/04
This report summarizes the results obtained in FY2020 at the Electron Linac Facility of the University of Tokyo. The radiolysis process of  -hexaoctyl nitrilotriacetamide (HONTA), which is expected to be used as an extractant in a separation process for minor actinides, diluted in dodecane was investigated by pulse radiolysis experiments. The radical cation and the triplet-excited state of HONTA were observed in the nanosecond time region. The transition from the radical cation to the triplet excited state was slowed down by adding electron scavengers, and further, the reactivity of the triplet excited state was also suppressed.
-hexaoctyl nitrilotriacetamide (HONTA), which is expected to be used as an extractant in a separation process for minor actinides, diluted in dodecane was investigated by pulse radiolysis experiments. The radical cation and the triplet-excited state of HONTA were observed in the nanosecond time region. The transition from the radical cation to the triplet excited state was slowed down by adding electron scavengers, and further, the reactivity of the triplet excited state was also suppressed.
 mesocrystal formation
mesocrystal formationLi, Z.*; Piankova, D.*; Yang, Y.*; Kumagai, Yuta; Zschiesche, H.*; Jonsson, M.*; Tarakina, N. V.*; Soroka, I. L.*
Angewandte Chemie; International Edition, 61(6), p.e202112204_1 - e202112204_9, 2022/02
Times Cited Count:4 Percentile:30.82(Chemistry, Multidisciplinary)The role of intermediate phases in CeO mesocrystal formation from aqueous Ce(III) solutions subjected to
mesocrystal formation from aqueous Ce(III) solutions subjected to  -radiation was studied. Radiolytically formed hydroxyl radicals convert soluble Ce(III) into less soluble Ce(IV). Transmission electron microscopy and X-ray diffraction studies of samples from different stages of the process allowed the identification of several stages in CeO
-radiation was studied. Radiolytically formed hydroxyl radicals convert soluble Ce(III) into less soluble Ce(IV). Transmission electron microscopy and X-ray diffraction studies of samples from different stages of the process allowed the identification of several stages in CeO mesocrystal evolution following the oxidation to Ce(IV): (1) formation of hydrated Ce(IV)-hydroxides, serving as intermediates in the liquid-to-solid phase transformation; (2) CeO
mesocrystal evolution following the oxidation to Ce(IV): (1) formation of hydrated Ce(IV)-hydroxides, serving as intermediates in the liquid-to-solid phase transformation; (2) CeO primary particle growth inside the intermediate phase; (3) alignment of the primary particles into "pre-mesocrystals" and subsequently to mesocrystals, guided by confinement of the amorphous intermediate phase and accompanied by the formation of mineral bridges. Further alignment of the obtained mesocrystals into supracrystals occurs upon slow drying, making it possible to form complex hierarchical architectures.
primary particle growth inside the intermediate phase; (3) alignment of the primary particles into "pre-mesocrystals" and subsequently to mesocrystals, guided by confinement of the amorphous intermediate phase and accompanied by the formation of mineral bridges. Further alignment of the obtained mesocrystals into supracrystals occurs upon slow drying, making it possible to form complex hierarchical architectures.
Kumagai, Yuta; Kimura, Atsushi*; Taguchi, Mitsumasa*; Watanabe, Masayuki
Radiation Physics and Chemistry, 191, p.109831_1 - 109831_8, 2022/02
Times Cited Count:0 Percentile:0.01(Chemistry, Physical)In this study, we investigated and compared the effects of a high-silica zeolite (HMOR) on the radiation-induced degradation of three aromatic chlorides, 2-chlorophenol (2-ClPh), 2-chloroaniline (2-ClAn), and 2-chlorobenzoic acid (2-ClBA), in order to examine its potential to reduce the influence of ions in water matrix in the irradiation treatment of water-soluble organic compounds. In the presence of ions reactive to radicals, the degradation of 2-ClPh in water was inhibited, but the combined use of HMOR much improved the degradation yield. This improvement was attributed to high performance of HMOR in adsorption of 2-ClPh. Similarly, HMOR was effective for adsorption of 2-ClAn and facilitated the 2-ClAn degradation by irradiation. In contrast, HMOR was poor at adsorption of 2-ClBA and consistently the degradation of 2-ClBA in the water-HMOR mixture was inhibited by the radical scavenger. These results demonstrate that HMOR can mitigate the influence of radical scavengers in water.
Kumagai, Yuta; Nagaishi, Ryuji; Kimura, Atsushi*; Taguchi, Mitsumasa*; Nishihara, Kenji; Yamagishi, Isao; Ogawa, Toru
Insights Concerning the Fukushima Daiichi Nuclear Accident, Vol.4; Endeavors by Scientists, p.37 - 45, 2021/10
Zeolite adsorbents are to be used for decontamination of radioactive water in Fukushima Dai-ichi Nuclear Power Station. Evaluation of hydrogen production by water radiolysis during decontamination is important for safe operation. Thus hydrogen production from the mixture of zeolite adsorbents and seawater was studied because seawater was urgently used as a coolant for the fuels. The hydrogen yield from the mixture decreased at a high weight fraction of zeolites. However, the measured yield was higher than the yield expected from the direct radiolysis of seawater in the mixture, which would decrease proportional to the weight fraction of seawater. The result suggests that the radiation energy deposited to zeolites was involved in the hydrogen formation. From the results, the hydrogen production rate was evaluated to be 3.6 mL/h per ton of radioactive water before decontamination. After the process, it was evaluated to be 1.5 L/h per ton of waste adsorbents due to the high dose rate.
 O
O decomposition at the U
decomposition at the U O
O surface in bicarbonate solution
surface in bicarbonate solutionMcGrady, J.; Kumagai, Yuta; Watanabe, Masayuki; Kirishima, Akira*; Akiyama, Daisuke*; Kitamura, Akira; Kimuro, Shingo
RSC Advances (Internet), 11(46), p.28940 - 28948, 2021/08
Times Cited Count:5 Percentile:38.19(Chemistry, Multidisciplinary) O
O ; Application of Raman imaging technique to uranium compound
; Application of Raman imaging technique to uranium compoundKusaka, Ryoji; Kumagai, Yuta; Yomogida, Takumi; Takano, Masahide; Watanabe, Masayuki; Sasaki, Takayuki*; Akiyama, Daisuke*; Sato, Nobuaki*; Kirishima, Akira*
Journal of Nuclear Science and Technology, 58(6), p.629 - 634, 2021/06
Times Cited Count:7 Percentile:66.68(Nuclear Science & Technology) -octylnitrilo-triacetamide (HONTA) complexes of americium and europium
-octylnitrilo-triacetamide (HONTA) complexes of americium and europiumToigawa, Tomohiro; Peterman, D. R.*; Meeker, D. S.*; Grimes, T. S.*; Zalupski, P. R.*; Mezyk, S. P.*; Cook, A. R.*; Yamashita, Shinichi*; Kumagai, Yuta; Matsumura, Tatsuro; et al.
Physical Chemistry Chemical Physics, 23(2), p.1343 - 1351, 2021/01
Times Cited Count:14 Percentile:81.5(Chemistry, Physical)The candidate An(III)/Ln(III) separation ligand hexa- -octylnitrilo-triacetamide (HONTA) was irradiated under envisioned SELECT (Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation) process conditions using a solvent test loop in conjunction with cobalt-60 gamma irradiation. We demonstrate that HONTA undergoes exponential decay with increasing gamma dose to produce a range of degradation products which have been identified and quantified by HPLC-ESI-MS/MS techniques. The combination of HONTA destruction and degradation product ingrowth, particularly dioctylamine, negatively impacts the extraction and back-extraction of both americium and europium ions. The loss of HONTA was attributed to its reaction with the solvent (
-octylnitrilo-triacetamide (HONTA) was irradiated under envisioned SELECT (Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation) process conditions using a solvent test loop in conjunction with cobalt-60 gamma irradiation. We demonstrate that HONTA undergoes exponential decay with increasing gamma dose to produce a range of degradation products which have been identified and quantified by HPLC-ESI-MS/MS techniques. The combination of HONTA destruction and degradation product ingrowth, particularly dioctylamine, negatively impacts the extraction and back-extraction of both americium and europium ions. The loss of HONTA was attributed to its reaction with the solvent ( -dodecane) radical cation of
-dodecane) radical cation of 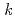 (HONTA + R
(HONTA + R ) = (7.61
) = (7.61  0.82)
0.82)  10
10 M
M s
s obtained by pulse radiolysis techniques. However, when this ligand is bound to either americium or europium ions, the observed
obtained by pulse radiolysis techniques. However, when this ligand is bound to either americium or europium ions, the observed  -dodecane radical cation kinetics increase by over an order of magnitude. This large reactivity increase to additional reaction pathways occurring upon metal-ion binding. Lastly nanosecond time-resolved measurements showed that both direct and indirect HONTA radiolysis yielded the short-lived (
-dodecane radical cation kinetics increase by over an order of magnitude. This large reactivity increase to additional reaction pathways occurring upon metal-ion binding. Lastly nanosecond time-resolved measurements showed that both direct and indirect HONTA radiolysis yielded the short-lived ( 100 ns) HONTA radical cation as well as a longer-lived (
100 ns) HONTA radical cation as well as a longer-lived ( s) HONTA triplet excited state. These HONTA species are important precursors to the suite of HONTA degradation products observed.
s) HONTA triplet excited state. These HONTA species are important precursors to the suite of HONTA degradation products observed.
Toigawa, Tomohiro; Tsubata, Yasuhiro; Kai, Takeshi; Furuta, Takuya; Kumagai, Yuta; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 39(1), p.74 - 89, 2021/00
Times Cited Count:2 Percentile:10.1(Chemistry, Multidisciplinary)Absorbed-dose estimation is essential for evaluation of the radiation feasibility of minor-actinide-separation processes. We propose a dose-evaluation method based on radiation permeability, with comparisons of heterogeneous structures seen in the solvent-extraction process, such as emulsions forming in the mixture of the organic and aqueous phases. A demonstration of radiation-energy-transfer simulation is performed with a focus on the minor-actinide-recovery process from high-level liquid waste with the aid of the Monte Carlo radiation-transport code PHITS. The simulation results indicate that the dose absorbed by the extraction solvent from alpha ray depends upon the emulsion structure, and that from beta and gamma ray depends upon the mixer-settler-apparatus size. Non-negligible contributions of well-permeable gamma rays were indicated in terms of the plant operation of the minor-actinide-separation process.
Toigawa, Tomohiro; Murayama, Rin*; Kumagai, Yuta; Yamashita, Shinichi*; Suzuki, Hideya; Ban, Yasutoshi; Matsumura, Tatsuro
UTNL-R-0501, p.24 - 25, 2020/12
This report summarizes the results obtained in FY2019 at Electron Linac Facility of University of Tokyo. The radiolysis process of a diglycolamide extractant, which is expected to be used in the separation process of minor actinides (MA), in dodecane and octanol solutions was investigated by pulse radiolysis. As a result, it was suggested that by adding alcohol, the decomposition process of the diglycolamide extractant was different from the decomposition processes in the single solvent of dodecane considered that the decomposition occurred via a radical cation species of the extractant.
 ssbauer, infrared, and laser-induced luminescence for classifying rare-earth minerals enriched in iron-rich deposits
ssbauer, infrared, and laser-induced luminescence for classifying rare-earth minerals enriched in iron-rich depositsAoyagi, Noboru; Nguyen, T. T.*; Kumagai, Yuta; Nguyen, T. V.*; Nakada, Masami; Segawa, Yukari; Nguyen, H. T.*; Le, B. T.*
ACS Omega (Internet), 5(13), p.7096 - 7105, 2020/04
Times Cited Count:3 Percentile:15.55(Chemistry, Multidisciplinary)