Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kawakita, Yukinobu; Otomo, Toshiya*; Ueno, Hiroki; Tsubota, Masami*; Kumara, L. S. R.*; Oshita, Hidetoshi*; Suzuya, Kentaro
no journal, ,
Ag (GeSe
(GeSe )
) ternary alloy exhibits an extensive compositional range of bulk glass forming and a rapid enhancement in ionic conductivity from 10
ternary alloy exhibits an extensive compositional range of bulk glass forming and a rapid enhancement in ionic conductivity from 10 to 10
to 10 S/cm of these glasses at Ag concentration of x=0.3 with increasing Ag content. Structural models of this material have already been proposed by reverse Monte Carlo simulation based on experimental structural data of neutron and X-ray diffractions and X-ray absorption fine structure. However, there observed micro-phase separation tendency in these glasses. This tendency might relate to formation of ionic conduction path in supercooled liquid on quenching process. The proposed models will be examined by structure factors over a wide Q range including relatively low momentum-transfer region which have been newly obtained by the total neutron scattering instrument installed at BL21 in MLF, J-PARC. This work was supported by NEDO under "Advanced Fundamental Research Project on Hydrogen Storage Materials".
S/cm of these glasses at Ag concentration of x=0.3 with increasing Ag content. Structural models of this material have already been proposed by reverse Monte Carlo simulation based on experimental structural data of neutron and X-ray diffractions and X-ray absorption fine structure. However, there observed micro-phase separation tendency in these glasses. This tendency might relate to formation of ionic conduction path in supercooled liquid on quenching process. The proposed models will be examined by structure factors over a wide Q range including relatively low momentum-transfer region which have been newly obtained by the total neutron scattering instrument installed at BL21 in MLF, J-PARC. This work was supported by NEDO under "Advanced Fundamental Research Project on Hydrogen Storage Materials".
Ueno, Hiroki; Kawakita, Yukinobu; Ohara, Koji*; Shimakura, Hironori; Tahara, Shuta*; Kumara, L. S. R.*; Yamaguchi, Hiroshi*; Yasunaga, Akinori*; Wakisaka, Yuiko*; Ito, Masayoshi*; et al.
no journal, ,
Bi-Zn system has a phase diagram with a miscibility gap where critical point is at Bi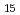 Zn
Zn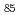 . It is known that concentration fluctuation increases on approaching to the miscibility gap. Earlier studies suggested that the miscibility gap is attributed to difference in atomic size between Bi and Zn. Recent developments of instrument and analysis technique enable us to deduce partial structures and demonstrate atomic configurations in liquid. We performed detailed analysis on medium-range structure in liquid Bi
. It is known that concentration fluctuation increases on approaching to the miscibility gap. Earlier studies suggested that the miscibility gap is attributed to difference in atomic size between Bi and Zn. Recent developments of instrument and analysis technique enable us to deduce partial structures and demonstrate atomic configurations in liquid. We performed detailed analysis on medium-range structure in liquid Bi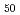 Zn
Zn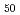 by using neutron diffraction data obtained by the two-axis diffractometer, HERMES, installed in JRR-3M, JAEA and, X-ray diffraction at BL08W beamline in SPring-8, Hyogo, Japan. 3D atomic structural model obtained by Revere Monte Carlo technique exhibits temperature evolution of concentration fluctuations in medium-range structure, which suggests that phase separation tendency appears even in the edge of the miscibility gap of liquid Bi-Zn.
by using neutron diffraction data obtained by the two-axis diffractometer, HERMES, installed in JRR-3M, JAEA and, X-ray diffraction at BL08W beamline in SPring-8, Hyogo, Japan. 3D atomic structural model obtained by Revere Monte Carlo technique exhibits temperature evolution of concentration fluctuations in medium-range structure, which suggests that phase separation tendency appears even in the edge of the miscibility gap of liquid Bi-Zn.
Kawakita, Yukinobu; Otomo, Toshiya*; Ueno, Hiroki; Tsubota, Masami*; Kumara, L. S. R.*; Oshita, Hidetoshi*; Suzuya, Kentaro; Takeda, Shinichi*
no journal, ,
Superionic glasses have attracted widespread interest in relation with their potential application as solid electrolytes in new devices. The interest in Ag (GeSe
(GeSe )
) ternary alloys derives from extensive compositional range of glass forming and rapid increase in conductivity at
ternary alloys derives from extensive compositional range of glass forming and rapid increase in conductivity at 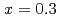 . Neutron diffraction measurements of Ag
. Neutron diffraction measurements of Ag (GeSe
(GeSe )
) glass have been performed over a wide Q range by utilizing the total neutron scattering instrument, NOVA, installed at BL21 in MLF, J-PARC. Since
glass have been performed over a wide Q range by utilizing the total neutron scattering instrument, NOVA, installed at BL21 in MLF, J-PARC. Since 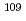 Ag is a good neutron absorber, NOVA instrument and method for data reduction can be examined by this sample. Structural model of this material has already been proposed by reverse Monte Carlo simulation based on experimental structural data. This structure model has been checked in consistency with new data in an extremely wide Q range. This work was supported by NEDO under "Advanced Fundamental Research Project on Hydrogen Storage Materials" (HydroStar).
Ag is a good neutron absorber, NOVA instrument and method for data reduction can be examined by this sample. Structural model of this material has already been proposed by reverse Monte Carlo simulation based on experimental structural data. This structure model has been checked in consistency with new data in an extremely wide Q range. This work was supported by NEDO under "Advanced Fundamental Research Project on Hydrogen Storage Materials" (HydroStar).