Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yamagishi, Isao; Odakura, Makoto; Ichige, Yoshiaki; Kuroha, Mitsuhiko; Takano, Masahide; Akabori, Mitsuo; Yoshioka, Masahiro*
Proceedings of 21st International Conference & Exhibition; Nuclear Fuel Cycle for a Low-Carbon Future (GLOBAL 2015) (USB Flash Drive), p.1113 - 1119, 2015/09
The characteristics of insoluble residues in fine suspension at the Rokkasho Reprocessing Plant were analyzed. The insoluble residues were washed with oxalic acid solution to dissolve zirconium molybdate residues. XRD profiles of unwashed residues showed the presence of a noble metal alloy, zirconium molybdate, and zirconia, but zirconium molybdate was not found after washing. More than 50% of the Sb-125 and Pu in thee residues was washed out as well. The noble metal alloy composed of Mo, Tc, Ru, Rh, and Pd occupied more than 90% of the total weight of 12 elements (Ca, Cr, Fe, Ni, Zr, Mo, Tc, Ru, Rh, Pd, Te, and U) found in the residues. In consideration of the chemical forms of 12 elements, the alloy-to-residue weight ratio was evaluated to be 64% and 78% with and without 18% of an unknown component, respectively.
Suguro, Toshiyasu; Notoya, Shin; Nishikawa, Yoshiaki*; Nakamura, Ryosuke*; Shibutani, Tomoki; Kuroha, Mitsuhiko; Kamei, Gento
JNC TN8430 2004-004, 27 Pages, 2005/01
In terms of safety assessment of TRU waste disposal, data on plutonium sorption on cementitious materials have been obtained by means of a static batch-type experiment. Because the repository condition will be reducing and be affected by considerable amount of nitrate, the authors carried out the experiments using ordinary portland cement (OPC) under the reducing (Na S
S O
O as added as reductant) and anoxic condition (O
as added as reductant) and anoxic condition (O
 1ppm) and solution of 0 to 0.5 M NaNO
1ppm) and solution of 0 to 0.5 M NaNO . Other experimental conditions are : liquid/solid (L/S) ratios ; 100 and 1000 mL g
. Other experimental conditions are : liquid/solid (L/S) ratios ; 100 and 1000 mL g , Initially aaded plutonium; 2.84
, Initially aaded plutonium; 2.84 10
10 M, Temperature; 25
M, Temperature; 25 5
5 C and Reaction times; 7, 14 and 28 days. The experimental results suggest that distribution coefficient (
C and Reaction times; 7, 14 and 28 days. The experimental results suggest that distribution coefficient (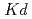 ) ranges 50 to 1000 mL g
) ranges 50 to 1000 mL g in case of L/S=100mL g
in case of L/S=100mL g . Similarly the
. Similarly the 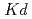 ranges, 100 to 10000 mL g
ranges, 100 to 10000 mL g at L/S=1000mL g
at L/S=1000mL g . These
. These 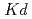 values tend to increase with lapsing reaction time. On the basis of these results, we recommend 50mL g
values tend to increase with lapsing reaction time. On the basis of these results, we recommend 50mL g as a conservative
as a conservative 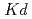 value of plutonium on OPC in a TRU waste repository condition.
value of plutonium on OPC in a TRU waste repository condition.
Notoya, Shin; Shibutani, Tomoki; Okazaki, M.*; Inui, Shinichi*; Kuroha, Mitsuhiko; Yui, Mikazu
JAERI-Conf 99-004, p.643 - 653, 1999/03
None
; ; Kuroha, Mitsuhiko; Yui, Mikazu; ;
JAERI-Conf 99-004, p.643 - 653, 1999/03
None
Kuroha, Mitsuhiko; ; Yamada, Kazuo; Yui, Mikazu; ;
JNC TN8410 98-001, 35 Pages, 1998/10
In order to study the migration behavior of plutonium from high-level vitrified waste in a deep repository condition, plutonium doped simulated high-level vitrified waste was prepared in a laboratory. Pu-doped glass was prepared with 1wt.% of PuO . The simulated high-level vitrified waste was crushed to less than 75
. The simulated high-level vitrified waste was crushed to less than 75 m and put into platinum crucible (
m and put into platinum crucible (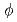 17 mm
17 mm  80 mm) with PuO
80 mm) with PuO powder sieved to less than 75
powder sieved to less than 75 m. The crucible was set into an electric furnace and the temperature was raised up to 1300
m. The crucible was set into an electric furnace and the temperature was raised up to 1300  C and kept for 2 hours. The melt was cooled down after annealing at 650
C and kept for 2 hours. The melt was cooled down after annealing at 650  C for 2 hours. The specimen was cut and homogeneity of Pu in Pu-doped glass was observed by
C for 2 hours. The specimen was cut and homogeneity of Pu in Pu-doped glass was observed by  -autoradiography. As a result of observation, plutonium was distributed homogeneously in the middle of the glass, but was distributed heterogeneously in the bottom of glass. And then the whole of the glass was crushed and melted again in the same way. Leaching experiments of Pu-doped glass and solubility experiments of Pu were carried out under aerobic conditions at room temperature. Leaching experiments of Pu-doped glass were carried out by using batch method with NaNO
-autoradiography. As a result of observation, plutonium was distributed homogeneously in the middle of the glass, but was distributed heterogeneously in the bottom of glass. And then the whole of the glass was crushed and melted again in the same way. Leaching experiments of Pu-doped glass and solubility experiments of Pu were carried out under aerobic conditions at room temperature. Leaching experiments of Pu-doped glass were carried out by using batch method with NaNO solution. The particle size of glass powder was under 75
solution. The particle size of glass powder was under 75 m and pH were adjusted by HNO
m and pH were adjusted by HNO and NaOH in the pH range 4-10. The experimental period was 151-340 days. Solubility experiments of Pu were also carried out from over saturation in 0.1M NaNO
and NaOH in the pH range 4-10. The experimental period was 151-340 days. Solubility experiments of Pu were also carried out from over saturation in 0.1M NaNO solution and pH were adjusted by HNO
solution and pH were adjusted by HNO and NaOH in the pH range 4-12. The experimental period was 42-233 days. These leachate were filtered through a 10,000 molecular weight cut off (MWCO) ultrafilter to separate solution and solid. These experiments were carried out in the teflon vessel. The Pu concentration in leachate was analyzed by
and NaOH in the pH range 4-12. The experimental period was 42-233 days. These leachate were filtered through a 10,000 molecular weight cut off (MWCO) ultrafilter to separate solution and solid. These experiments were carried out in the teflon vessel. The Pu concentration in leachate was analyzed by  -spectrometry. The Pu concentration from the Pu-doped glass was around 10
-spectrometry. The Pu concentration from the Pu-doped glass was around 10 mol/l in the pH range 4-6, and decreased to 10
mol/l in the pH range 4-6, and decreased to 10 mol/l with increasing pH. The leaching ...
mol/l with increasing pH. The leaching ...
Shibutani, Tomoki; Yui, Mikazu; Kuroha, Mitsuhiko
Proceedings of Migration '97, 0 Pages, 1996/00
None
Saito, Makoto; Yamada, Kazuo; Kitano, Mitsuaki; Kuroha, Mitsuhiko; Seimiya, Hiroshi
PNC TN8410 92-056, 43 Pages, 1992/03
None
Seimiya, Hiroshi; Yamada, Kazuo; Kuroha, Mitsuhiko; Saito, Makoto; Tomikawa, Hirofumi; Saito, Toru*; Hagiya, Shinichi*
PNC TN8410 90-082, 90 Pages, 1990/09
None
Sasaki, Noriaki; Yusa, Yasuhisa; Yamada, Kazuo; Nodaka, Masayuki*; ; Kawamura, Kazuhiro; Miyahara, Kaname; Arai, Takashi; Kamei, Gento; Hirose, Ikuro; et al.
PNC TN8440 88-018, 170 Pages, 1988/12
None
Odakura, Makoto; Ichige, Yoshiaki; Kuroha, Mitsuhiko; Yamagishi, Isao; Ishihara, Miho; Fukui, Toshiki*; Yoshioka, Masahiro*
no journal, ,
no abstracts in English
Fujiwara, Kenso; Odakura, Makoto; Kuroha, Mitsuhiko; Kohara, Yukitoshi*; Kikuchi, Hiroshi*
no journal, ,
The purpose of this study is to alteration-phase formation and associated elemental release furing aqueous corrosion of high-level waste(HLW) glass. Static corrosion tests were formed at high temperature (90, 120 degrees) alkali (pH 11, 12, 13) condition. Crystalline alteration-phase formed in the corroded glass were made. Most of Cs in the glass is retained in the alteration-phases into amorphous phases and pollucite.