Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Li, N.*; Sun, Y.*; Nakajima, Kunihisa; Kurosaki, Ken*
Journal of Nuclear Science and Technology, 61(3), p.343 - 353, 2024/03
Times Cited Count:1 Percentile:23.64(Nuclear Science & Technology)During the Fukushima Daiichi nuclear power plant (1F) accident, an overwhelming amount of the cesium remaining in the pressure vessel could have been deposited onto 304 stainless steel (SS304) steam separators and dryers, both with large surface areas. During 1F's decommissioning, the deposited cesium is a safety hazard as it can generate radioactive dust. However, the cohesive and adhesive strengths of CsOH-chemisorbed oxide scales are yet to be defined. In this study, we investigated how CsOH-chemisorption affects the cohesive and adhesive strengths between oxide scales and SS304 substrates with a scratch tester. The scratch test results revealed that the cohesive strengths of the oxide scales decreased after CsOH-chemisorption, while adhesive failure could not be reached.
 Si
Si O
O
Suzuki, Eriko; Nakajima, Kunihisa; Osaka, Masahiko; Oishi, Yuji*; Muta, Hiroaki*; Kurosaki, Ken*
Journal of Nuclear Science and Technology, 57(7), p.852 - 857, 2020/07
Times Cited Count:5 Percentile:42.20(Nuclear Science & Technology)The low temperature heat capacity of Cs Si
Si O
O , which is one of the cesium chemisorbed compounds onto stainless steel during severe accident of the light water nuclear reactor, was experimentally determined for the first time in the temperature range of 1.9 - 302 K. The experimentally determined heat capacity,
, which is one of the cesium chemisorbed compounds onto stainless steel during severe accident of the light water nuclear reactor, was experimentally determined for the first time in the temperature range of 1.9 - 302 K. The experimentally determined heat capacity, 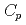
 (298.15K), and the standard entropy,
(298.15K), and the standard entropy, 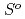 (298.15K), were 249.4
(298.15K), were 249.4  1.1 J K
1.1 J K mol
mol and 322.1
and 322.1  1.3 J K
1.3 J K mol
mol , respectively. The standard Gibbs energy of formation of Cs
, respectively. The standard Gibbs energy of formation of Cs Si
Si O
O at high temperatures,
at high temperatures, 

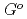 (
( ), were reevaluated by using the presently obtained
), were reevaluated by using the presently obtained 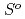 (298.15K) and the previously reported experimental results of the standard enthalpy of formation,
(298.15K) and the previously reported experimental results of the standard enthalpy of formation, 

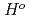 (298.15K), and the standard enthalpy increments at high temperatures,
(298.15K), and the standard enthalpy increments at high temperatures, 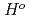 (
( )-
)-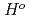 (298.15K).
(298.15K).
Mohamad, A.*; Nakajima, Kunihisa; Suzuki, Eriko; Miwa, Shuhei; Osaka, Masahiko; Oishi, Yuji*; Muta, Hiroaki*; Kurosaki, Ken*
Proceedings of International Topical Workshop on Fukushima Decommissioning Research (FDR 2019) (Internet), 4 Pages, 2019/05
In the accident of Fukushima Daiichi Nuclear Power Station, formation of a volatile SrCl could have occurred by the sea-water injection into the core. This can cause the release of non-volatile group Sr from the fuel to induce chemical reactions with reactor structural materials, such as stainless steel and Zircaloy (Zry) cladding. Such reactions could cause the changes in distribution of Sr in the reactor. Chemical reactions between Sr species and Zry were therefore investigated experimentally. As the result, it can be said that Sr vapor species were chemically trapped right after the release from fuel. This trapping effect of Sr by Zry-cladding implies a possibility of preferable Sr retention in the oxide phase of debris.
could have occurred by the sea-water injection into the core. This can cause the release of non-volatile group Sr from the fuel to induce chemical reactions with reactor structural materials, such as stainless steel and Zircaloy (Zry) cladding. Such reactions could cause the changes in distribution of Sr in the reactor. Chemical reactions between Sr species and Zry were therefore investigated experimentally. As the result, it can be said that Sr vapor species were chemically trapped right after the release from fuel. This trapping effect of Sr by Zry-cladding implies a possibility of preferable Sr retention in the oxide phase of debris.
 -based simulated fuel containing CsI
-based simulated fuel containing CsITakamatsu, Yuki*; Ishii, Hiroto*; Oishi, Yuji*; Muta, Hiroaki*; Yamanaka, Shinsuke*; Suzuki, Eriko; Nakajima, Kunihisa; Miwa, Shuhei; Osaka, Masahiko; Kurosaki, Ken*
Nihon Genshiryoku Gakkai Wabun Rombunshi, 17(3/4), p.106 - 110, 2018/12
In order to establish the synthesis method of simulated fuel contacting Cesium (Cs) which is required for the evaluation of physical/chemical characteristics in fuel and release behavior of Cs, sintering tests of the cerium dioxide (CeO ) based simulated fuels containing Cesium iodide (CsI) are performed by using spark plasma sintering (SPS) method. The sintered CeO
) based simulated fuels containing Cesium iodide (CsI) are performed by using spark plasma sintering (SPS) method. The sintered CeO pellets with homogeneous distribution of several micro meter of CsI spherical precipitates were successfully obtained by optimizing SPS conditions.
pellets with homogeneous distribution of several micro meter of CsI spherical precipitates were successfully obtained by optimizing SPS conditions.
Muta, Hiroaki*; Nishikane, Ryoji*; Ando, Yusuke*; Matsunaga, Junji*; Sakamoto, Kan*; Harjo, S.; Kawasaki, Takuro; Oishi, Yuji*; Kurosaki, Ken*; Yamanaka, Shinsuke*
Journal of Nuclear Materials, 500, p.145 - 152, 2018/03
Times Cited Count:17 Percentile:79.90(Materials Science, Multidisciplinary)Tanaka, Kosuke; Sato, Isamu; Hirosawa, Takashi; Kurosaki, Ken*; Muta, Hiroaki*; Yamanaka, Shinsuke*
Journal of Nuclear Science and Technology, 52(10), p.1285 - 1289, 2015/10
Times Cited Count:2 Percentile:16.04(Nuclear Science & Technology)Polycrystalline specimens of americium-containing barium plutonate have been prepared by mixing the appropriate amounts of (Pu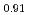 Am
Am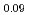 )O
)O and BaCO
and BaCO powders followed by reacting and sintering at 1600 K under the flowing gas atmosphere of dry-air. The sintered specimens had a single phase of orthorhombic perovskite structure and were crack-free. The elastic moduli were determined from the longitudinal and shear sound velocities. The Debye temperature was also determined from the sound velocities and lattice parameter measurements. The thermal conductivity was calculated from the measured density at room temperature, literature values of heat capacity, and thermal diffusivity measured by laser flash method in vacuum. The thermal conductivity of americium-containing barium plutonate was roughly independent of the temperature and was almost the same magnitude as that of BaPuO
powders followed by reacting and sintering at 1600 K under the flowing gas atmosphere of dry-air. The sintered specimens had a single phase of orthorhombic perovskite structure and were crack-free. The elastic moduli were determined from the longitudinal and shear sound velocities. The Debye temperature was also determined from the sound velocities and lattice parameter measurements. The thermal conductivity was calculated from the measured density at room temperature, literature values of heat capacity, and thermal diffusivity measured by laser flash method in vacuum. The thermal conductivity of americium-containing barium plutonate was roughly independent of the temperature and was almost the same magnitude as that of BaPuO and BaUO
and BaUO .
.

Tanaka, Kosuke; Tokushima, Kazuyuki*; Kurosaki, Ken*; Oishi, Yuji*; Muta, Hiroaki*; Yamanaka, Shinsuke*
Journal of Nuclear Materials, 443(1-3), p.218 - 221, 2013/11
Times Cited Count:0 Percentile:0.00(Materials Science, Multidisciplinary)Polycrystalline specimens of barium uranate, BaUO , were prepared and several properties such as the thermal expansion coefficient, elastic moduli, thermal conductivity, and Debye temperature were evaluated.
, were prepared and several properties such as the thermal expansion coefficient, elastic moduli, thermal conductivity, and Debye temperature were evaluated.
Tanaka, Kosuke; Sato, Isamu; Hirosawa, Takashi; Kurosaki, Ken*; Muta, Hiroaki*; Yamanaka, Shinsuke*
Journal of Nuclear Materials, 422(1-3), p.163 - 166, 2012/03
Times Cited Count:11 Percentile:60.79(Materials Science, Multidisciplinary)Polycrystalline specimens of strontium plutonate, SrPuO , have been prepared by mixing the appropriate amounts of PuO
, have been prepared by mixing the appropriate amounts of PuO and SrCO
and SrCO powders followed by reacting and sintering at 1600 K under the flowing gas atmosphere of dry-air. The sintered specimens had a single phase of orthorhombic perovskite structure and were crack-free. The elastic moduli of SrPuO
powders followed by reacting and sintering at 1600 K under the flowing gas atmosphere of dry-air. The sintered specimens had a single phase of orthorhombic perovskite structure and were crack-free. The elastic moduli of SrPuO were determined from the longitudinal and shear sound velocities. The Debye temperature was also determined from the sound velocities and lattice parameter measurements. The thermal conductivity of SrPuO
were determined from the longitudinal and shear sound velocities. The Debye temperature was also determined from the sound velocities and lattice parameter measurements. The thermal conductivity of SrPuO was calculated from the measured density at room temperature, literature values of heat capacity, and thermal diffusivity measured by laser flash method in vacuum.
was calculated from the measured density at room temperature, literature values of heat capacity, and thermal diffusivity measured by laser flash method in vacuum.
Tanaka, Kosuke; Osaka, Masahiko; Miwa, Shuhei; Hirosawa, Takashi; Kurosaki, Ken*; Muta, Hiroaki*; Uno, Masayoshi*; Yamanaka, Shinsuke*
Journal of Nuclear Materials, 420(1-3), p.207 - 212, 2012/01
Times Cited Count:6 Percentile:40.88(Materials Science, Multidisciplinary)In order to investigate the effect on fuel thermophysical properties when adding americium and selected fission products to uranium-plutonium mixed fuel, simulated low decontamination MOX fuel with high burn-ups to 250 GWd/t, has been prepared and subjected to characterization tests, elastic moduli measurements, melting temperature measurement.
Osaka, Masahiko; Konashi, Kenji*; Hayashi, Hirokazu; Li, D.*; Homma, Yoshiya*; Yamamura, Tomoo*; Sato, Isamu; Miwa, Shuhei; Sekimoto, Shun*; Kubota, Takumi*; et al.
Proceedings of International Conference on Toward and Over the Fukushima Daiichi Accident (GLOBAL 2011) (CD-ROM), 5 Pages, 2011/12
Summer schools for future experts have successfully been completed under Japan Actinide Network (J-ACTINET) for the purpose of development of human resources who are expected to be engaged in every areas of actinide-research/engineering. The first summer school was held in Ibaraki-area in August 2009, followed by the second one in Kansai-area in August 2010. Two summer schools have focused on actual experiences of actinides in actinide-research fields for university students and young researchers/engineers as an introductory course of actinide-researches. Several quasi actinide-handling experiences at the actinide-research fields have attracted attentions of participants at the first school in Ibaraki-area. The actual experiments using actinides-containing solutions have been carried out at the second school in Kansai-area. Future summer schools will be held every year for the sustainable human resource development in various actinide-research fields.
 at temperatures from 300 to 1500 K
at temperatures from 300 to 1500 KTanaka, Kosuke; Sato, Isamu; Hirosawa, Takashi; Kurosaki, Ken*; Muta, Hiroaki*; Yamanaka, Shinsuke*
Journal of Nuclear Materials, 414(2), p.316 - 319, 2011/07
Times Cited Count:24 Percentile:84.05(Materials Science, Multidisciplinary)Polycrystalline specimens of barium plutonate, BaPuO , have been prepared by mixing the appropriate amounts of PuO
, have been prepared by mixing the appropriate amounts of PuO and BaCO
and BaCO followed by reacting and sintering at 1600 K under the flowing gas atmosphere of dry-air. The thermal conductivity of BaPuO
followed by reacting and sintering at 1600 K under the flowing gas atmosphere of dry-air. The thermal conductivity of BaPuO was almost the same magnitude as that of BaUO
was almost the same magnitude as that of BaUO .
.
 Ce
Ce )O
)O
Osaka, Masahiko; Miwa, Shuhei; Kurosaki, Ken*; Uno, Masayoshi*; Yamanaka, Shinsuke*
Journal of Nuclear Materials, 408(3), p.285 - 288, 2011/01
Times Cited Count:3 Percentile:24.69(Materials Science, Multidisciplinary)Oxygen non-stoichiometry in (Th Ce
Ce )O
)O were investigated from a viewpoint of features of Ce reduction in several oxide solid solutions. Oxygen non-stoichiometry was experimentally determined by means of thermogravimetric analysis as a function of oxygen potential at 1173, 1273 and 1373 K. The similar features of isotherms of oxygen non-stoichiometry in (Th
were investigated from a viewpoint of features of Ce reduction in several oxide solid solutions. Oxygen non-stoichiometry was experimentally determined by means of thermogravimetric analysis as a function of oxygen potential at 1173, 1273 and 1373 K. The similar features of isotherms of oxygen non-stoichiometry in (Th Ce
Ce )O
)O with those in actinide and/or lanthanide-oxygen dioxides were observed. Oxygen non-stoichiometry in (Th
with those in actinide and/or lanthanide-oxygen dioxides were observed. Oxygen non-stoichiometry in (Th Ce
Ce )O
)O were compared with those of CeO
were compared with those of CeO and (U
and (U Ce
Ce )O
)O . It was concluded that features of Ce reduction have some relationships with defect forms and their transformations in the solid solutions.
. It was concluded that features of Ce reduction have some relationships with defect forms and their transformations in the solid solutions.
 -O (
-O ( = Mo or U) ternary compounds
= Mo or U) ternary compoundsTokushima, Kazuyuki*; Tanaka, Kosuke; Kurosaki, Ken*; Gima, Hiromichi*; Muta, Hiroaki*; Uno, Masayoshi*; Yamanaka, Shinsuke*
Materials Research Society Symposium Proceedings, Vol.1215, p.151 - 156, 2010/10
The thermal diffusivities of Cs MoO
MoO and Cs
and Cs UO
UO using samples fabricated by hot press and SPS techniques were measured by a laser flash method in the range from room temperature to 823 K for Cs
using samples fabricated by hot press and SPS techniques were measured by a laser flash method in the range from room temperature to 823 K for Cs MoO
MoO and to 900 K for Cs
and to 900 K for Cs UO
UO . The thermal conductivities of these cesium ternary oxides were quite low compared with UO
. The thermal conductivities of these cesium ternary oxides were quite low compared with UO and MOX fuel. This is consistent with previous findings. These results would be useful for evaluating the thermal performance of MOX fuels at the high burn-up region in the fast reactors.
and MOX fuel. This is consistent with previous findings. These results would be useful for evaluating the thermal performance of MOX fuels at the high burn-up region in the fast reactors.
Tanaka, Kosuke; Osaka, Masahiko; Kurosaki, Ken*; Muta, Hiroaki*; Uno, Masayoshi*; Yamanaka, Shinsuke*
Materials Research Society Symposium Proceedings, Vol.1215, p.95 - 100, 2010/10
In order to investigate the effect of MAs and FP addition on the oxygen potential of MOX fuels, thermogravimetric analysis (TGA) were carried out. MOX fuels with Am and 26 kinds of fission product elements (FPs), simulating low-decontamination MOX fuel and high burn-up to 250 GWd/t, were prepared by a conventional powder metallurgical route in a glove box. The oxygen potentials for simulating low-decontamination MOX fuels were higher than the fuels without FPs and increased positively with increasing simulated burn-up.
 Ce
Ce )O
)O
Osaka, Masahiko; Tanaka, Kosuke; Miwa, Shuhei; Kurosaki, Ken*; Uno, Masayoshi*; Yamanaka, Shinsuke*
Materials Research Society Symposium Proceedings, Vol.1215, p.199 - 203, 2010/10
Oxygen potentials of (Th Ce
Ce )O
)O were experimentally determined by means of thermogravimetric analysis as a function of non-stoichiometry at 1173 and 1273 K. Oxygen potentials of (Th
were experimentally determined by means of thermogravimetric analysis as a function of non-stoichiometry at 1173 and 1273 K. Oxygen potentials of (Th Ce
Ce )O
)O at each temperature increased with increase of O/M ratio (= 2-x) and steep increases of the oxygen potentials toward O/M = 2 were observed. These characteristics are typical for non-stoichiometric fluorite-type actinides dioxides. The oxygen potentials of (Th
at each temperature increased with increase of O/M ratio (= 2-x) and steep increases of the oxygen potentials toward O/M = 2 were observed. These characteristics are typical for non-stoichiometric fluorite-type actinides dioxides. The oxygen potentials of (Th Ce
Ce )O
)O were similar to those of CeOO
were similar to those of CeOO when they were plotted as a function of average Ce valence.
when they were plotted as a function of average Ce valence.
Kurosaki, Ken*; Tanaka, Kosuke; Osaka, Masahiko; Oishi, Yuji*; Muta, Hiroaki*; Uno, Masayoshi*; Yamanaka, Shinsuke*
Proceedings of Joint International Conference of 7th Supercomputing in Nuclear Application and 3rd Monte Carlo (SNA + MC 2010) (USB Flash Drive), 4 Pages, 2010/10
It is important to understand the behavior of fission products and actinides under irradiation. In the present study, the chemical states of fission products and actinides in irradiated oxide fuels were evaluated by both thermodynamic equilibrium calculation and post-irradiation examination.
Adachi, Jun*; Kurosaki, Ken*; Uno, Masayoshi*; Yamanaka, Shinsuke*; Takano, Masahide; Akabori, Mitsuo; Minato, Kazuo
Journal of Nuclear Materials, 384(1), p.6 - 11, 2009/01
Times Cited Count:14 Percentile:65.35(Materials Science, Multidisciplinary)The indentation hardness, Vickers hardness, fracture toughness, and Young's modulus of polycrystalline uranium monontride (UN) at sub-microscale and macroscale were evaluated by an indentation test, Vickers hardness test, and the ultrasonic pulse echo method. The Young's modulus and Vickers hardness were in good agreement with the literature values. The fracture toughness of UN was about three times that of UO . In addition, we revealed the indentation size effect on the indentation hardness of UN.
. In addition, we revealed the indentation size effect on the indentation hardness of UN.
Katayama, Masahito*; Adachi, Jun*; Kurosaki, Ken*; Uno, Masayoshi*; Miwa, Shuhei; Osaka, Masahiko; Tanaka, Kenya; Yamanaka, Shinsuke*
Materials Research Society Symposium Proceedings, Vol.1043, p.190 - 195, 2008/00
The molecular dynamics (MD) calculation was performed on minor actinides (MA)-containing mixed oxide (MOX) fuels, (U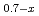 Pu
Pu MA
MA )O
)O (x = 0, 0.016, 0.03, 0.05, 0.1, 0.15), and the lattice parameter, heat capacity and thermal conductivity were evaluated. The calculated thermal conductivity data are comparable in (U
(x = 0, 0.016, 0.03, 0.05, 0.1, 0.15), and the lattice parameter, heat capacity and thermal conductivity were evaluated. The calculated thermal conductivity data are comparable in (U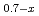 Pu
Pu MA
MA )O
)O at any temperature and any x value, indicating that the thermal conductivity of MA-containing MOX fuels is scarcely influenced by adding MA up to 15 %. The present study shows that the MD calculation can be usefully applied to determine the thermophysical properties of MA-containing MOX fuels.
at any temperature and any x value, indicating that the thermal conductivity of MA-containing MOX fuels is scarcely influenced by adding MA up to 15 %. The present study shows that the MD calculation can be usefully applied to determine the thermophysical properties of MA-containing MOX fuels.
Osaka, Masahiko; Adachi, Jun*; Kurosaki, Ken*; Uno, Masayoshi*; Yamanaka, Shinsuke*
Journal of Nuclear Science and Technology, 44(12), p.1543 - 1549, 2007/12
Times Cited Count:12 Percentile:62.34(Nuclear Science & Technology)no abstracts in English
 with addition of 9% Am
with addition of 9% AmMiwa, Shuhei; Osaka, Masahiko; Yoshimochi, Hiroshi; Tanaka, Kenya; Kurosaki, Ken*; Uno, Masayoshi*; Yamanaka, Shinsuke*
Journal of Alloys and Compounds, 444-445, p.610 - 613, 2007/10
Times Cited Count:14 Percentile:60.50(Chemistry, Physical)no abstracts in English