Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yakushev, A.*; Lens, L.*; D llmann, Ch. E.*; Khuyagbaatar, J.*; J
llmann, Ch. E.*; Khuyagbaatar, J.*; J ger, E.*; Krier, J.*; Runke, J.*; Albers, H. M.*; Asai, Masato; Block, M.*; et al.
ger, E.*; Krier, J.*; Runke, J.*; Albers, H. M.*; Asai, Masato; Block, M.*; et al.
Frontiers in Chemistry (Internet), 10, p.976635_1 - 976635_11, 2022/08
Times Cited Count:19 Percentile:80.70(Chemistry, Multidisciplinary)Flerovium (Fl, element 114) is the heaviest element chemically studied so far. The first chemical experiment on Fl suggested that Fl is a noble-gas-like element, while the second studies suggested that Fl has a volatile-metal-like character. To obtain more reliable conclusion, we performed further experimental studies on Fl adsorption behavior on Si oxide and gold surfaces. The present results suggest that Fl is highly volatile and less reactive than the volatile metal, Hg, but has higher reactivity than the noble gas, Rn.
Khuyagbaatar, J.*; Yakushev, A.*; D llmann, Ch. E.*; Ackermann, D.*; Andersson, L.-L.*; Asai, Masato; Block, M.*; Boll, R. A.*; Brand, H.*; Cox, D. M.*; et al.
llmann, Ch. E.*; Ackermann, D.*; Andersson, L.-L.*; Asai, Masato; Block, M.*; Boll, R. A.*; Brand, H.*; Cox, D. M.*; et al.
Physical Review C, 102(6), p.064602_1 - 064602_9, 2020/12
Times Cited Count:76 Percentile:99.03(Physics, Nuclear)A search for production of the superheavy elements with atomic numbers 119 and 120 was performed in the  Ti+
Ti+ Bk and
Bk and  Ti+
Ti+ Cf fusion-evaporation reactions, respectively, at the gas-filled recoil separator TASCA. Over four months of irradiation, neither was detected at cross-section sensitivity levels of 65 and 200 fb, respectively. The non-observation of elements 119 and 120 is discussed within the concept of fusion-evaporation reactions including various theoretical predictions on the fission-barrier heights of superheavy nuclei in the region of the island of stability.
Cf fusion-evaporation reactions, respectively, at the gas-filled recoil separator TASCA. Over four months of irradiation, neither was detected at cross-section sensitivity levels of 65 and 200 fb, respectively. The non-observation of elements 119 and 120 is discussed within the concept of fusion-evaporation reactions including various theoretical predictions on the fission-barrier heights of superheavy nuclei in the region of the island of stability.
 Ca+
Ca+ Bk leading to formation of the element Ts (Z=117)
Bk leading to formation of the element Ts (Z=117)Khuyagbaatar, J.*; Yakushev, A.*; D llmann, Ch. E.*; Ackermann, D.*; Andersson, L.-L.*; Asai, Masato; Block, M.*; Boll, R. A.*; Brand, H.*; Cox, D. M.*; et al.
llmann, Ch. E.*; Ackermann, D.*; Andersson, L.-L.*; Asai, Masato; Block, M.*; Boll, R. A.*; Brand, H.*; Cox, D. M.*; et al.
Physical Review C, 99(5), p.054306_1 - 054306_16, 2019/05
Times Cited Count:33 Percentile:92.86(Physics, Nuclear)We have performed an experiment to synthesize the element 117 (Ts) with the  Ca+
Ca+ Bk fusion reaction. Four
Bk fusion reaction. Four  -decay chains attributed to the element 117 were observed. Two of them were long decay chains which can be assigned to the one originating from the
-decay chains attributed to the element 117 were observed. Two of them were long decay chains which can be assigned to the one originating from the  decay of
decay of 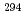 Ts. The other two were short decay chains which are consistent with the one originating from the
Ts. The other two were short decay chains which are consistent with the one originating from the  decay of
decay of 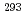 Ts. We have compared the present results with the literature data, and found that our present results mostly confirmed the literature data, leading to the firm confirmation of the synthesis of the element 117.
Ts. We have compared the present results with the literature data, and found that our present results mostly confirmed the literature data, leading to the firm confirmation of the synthesis of the element 117.
 and Au surfaces in preparation for chemical investigations on Cn, Nh, and Fl at TASCA
and Au surfaces in preparation for chemical investigations on Cn, Nh, and Fl at TASCALens, L.*; Yakushev, A.*; D llmann, Ch. E.*; Asai, Masato; Ballof, J.*; Block, M.*; David, H. M.*; Despotopulos, J.*; Di Nitto, A.*; Eberhardt, K.*; et al.
llmann, Ch. E.*; Asai, Masato; Ballof, J.*; Block, M.*; David, H. M.*; Despotopulos, J.*; Di Nitto, A.*; Eberhardt, K.*; et al.
Radiochimica Acta, 106(12), p.949 - 962, 2018/12
Times Cited Count:11 Percentile:68.03(Chemistry, Inorganic & Nuclear)Online gas-solid adsorption studies with single atom quantities of Hg, Tl, and Pb on SiO and Au surfaces were carried out using short-lived radioisotopes with half-lives in the range of 4-49 s. This is a model study to measure adsorption enthalpies of superheavy elements Cn, Nh, and Fl. The short-lived isotopes were produced and separated by the gas-filled recoil separator TASCA at GSI. The products were stopped in He gas, and flushed into gas chromatography columns made of Si detectors whose surfaces were covered by SiO
and Au surfaces were carried out using short-lived radioisotopes with half-lives in the range of 4-49 s. This is a model study to measure adsorption enthalpies of superheavy elements Cn, Nh, and Fl. The short-lived isotopes were produced and separated by the gas-filled recoil separator TASCA at GSI. The products were stopped in He gas, and flushed into gas chromatography columns made of Si detectors whose surfaces were covered by SiO or Au. The short-lived Tl and Pb were successfully measured by the Si detectors with the SiO
or Au. The short-lived Tl and Pb were successfully measured by the Si detectors with the SiO surface at room temperature. On the other hand, the Hg did not adsorb on the SiO
surface at room temperature. On the other hand, the Hg did not adsorb on the SiO surface, but adsorbed on the Au surface. The results demonstrated that the adsorption properties of short-lived Hg, Tl, and Pb could be studied with this setup, and that this method is applicable to the experiment for Cn, Nh, and Fl.
surface, but adsorbed on the Au surface. The results demonstrated that the adsorption properties of short-lived Hg, Tl, and Pb could be studied with this setup, and that this method is applicable to the experiment for Cn, Nh, and Fl.
 -decaying millisecond isomeric state in
-decaying millisecond isomeric state in 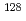 Cd
Cd
Jungclaus, A.*; Grawe, H.*; Nishimura, Shunji*; Doornenbal, P.*; Lorusso, G.*; Simpson, G. S.*; S derstr
derstr m, P.-A.*; Sumikama, Toshiyuki*; Taprogge, J.*; Xu, Z. Y.*; et al.
m, P.-A.*; Sumikama, Toshiyuki*; Taprogge, J.*; Xu, Z. Y.*; et al.
Physics Letters B, 772, p.483 - 488, 2017/09
Times Cited Count:8 Percentile:52.04(Astronomy & Astrophysics)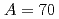 isobars from the
isobars from the 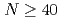 island of inversion
island of inversionMorales, A. I.*; Benzoni, G.*; Watanabe, H.*; Tsunoda, Yusuke*; Otsuka, T.*; Nishimura, Shunji*; Browne, F.*; Daido, R.*; Doornenbal, P.*; Fang, Y.*; et al.
Physics Letters B, 765, p.328 - 333, 2017/02
Times Cited Count:38 Percentile:92.42(Astronomy & Astrophysics)Eichler, R.*; Asai, Masato; Brand, H.*; Chiera, N. M.*; Di Nitto, A.*; Dressler, R.*; D llmann, Ch. E.*; Even, J.*; Fangli, F.*; Goetz, M.*; et al.
llmann, Ch. E.*; Even, J.*; Fangli, F.*; Goetz, M.*; et al.
EPJ Web of Conferences, 131, p.07005_1 - 07005_7, 2016/12
Times Cited Count:3 Percentile:71.33(Chemistry, Inorganic & Nuclear)In recent years gas-phase chemical studies assisted by physical pre-separation allowed for the productions and investigations of fragile single molecular species of superheavy elements. The latest highlight is the formation of very volatile hexacarbonyl compound of element 106, Sg(CO) . Following this success, second-generation experiments were performed to measure the first bond dissociation energy between the central metal atom and the surrounding ligand. The method using a tubular decomposition reactor was developed and successfully applied to short-lived Mo(CO)
. Following this success, second-generation experiments were performed to measure the first bond dissociation energy between the central metal atom and the surrounding ligand. The method using a tubular decomposition reactor was developed and successfully applied to short-lived Mo(CO) , W(CO)
, W(CO) , and Sg(CO)
, and Sg(CO) .
.
 -fission and recoil-
-fission and recoil- -
- -fission events observed in the reaction
-fission events observed in the reaction  Ca +
Ca +  Am
AmForsberg, U.*; Rudolph, D.*; Andersson, L.-L.*; Di Nitto, A.*; D llmann, Ch. E.*; Fahlander, C.*; Gates, J. M.*; Golubev, P.*; Gregorich, K. E.*; Gross, C. J.*; et al.
llmann, Ch. E.*; Fahlander, C.*; Gates, J. M.*; Golubev, P.*; Gregorich, K. E.*; Gross, C. J.*; et al.
Nuclear Physics A, 953, p.117 - 138, 2016/09
Times Cited Count:54 Percentile:95.07(Physics, Nuclear)Alpha-decay chains observed in the element-115 production reactions of  Ca +
Ca +  Am were investigated using a new data set consisting of our recently reported data obtained at GSI and previously reported ones at Dubna and LBNL. Short decay chains of recoil-
Am were investigated using a new data set consisting of our recently reported data obtained at GSI and previously reported ones at Dubna and LBNL. Short decay chains of recoil- -(
-( )-fission type, fourteen of which were observed, and some of which were interpreted as the 2-neutron evaporation products
)-fission type, fourteen of which were observed, and some of which were interpreted as the 2-neutron evaporation products 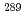 Mc, have been reassigned. It is plausible that most of them were assigned to the 3-neutron evaporation products
Mc, have been reassigned. It is plausible that most of them were assigned to the 3-neutron evaporation products 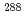 Mc whose decay chain would on the way have branches of EC decays followed by fission.
Mc whose decay chain would on the way have branches of EC decays followed by fission.
 decay of semi-magic
decay of semi-magic 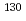 Cd; Revision and extension of the level scheme of
Cd; Revision and extension of the level scheme of 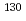 In
InJungclaus, A.*; Grawe, H.*; Nishimura, Shunji*; Doornenbal, P.*; Lorusso, G.*; Simpson, G. S.*; S derstr
derstr m, P. A.*; Sumikama, Toshiyuki*; Taprogge, J.*; Xu, Z. Y.*; et al.
m, P. A.*; Sumikama, Toshiyuki*; Taprogge, J.*; Xu, Z. Y.*; et al.
Physical Review C, 94(2), p.024303_1 - 024303_8, 2016/08
Times Cited Count:19 Percentile:75.93(Physics, Nuclear) rays emitted from excited states south-east of
rays emitted from excited states south-east of 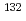 Sn; The
Sn; The  g
g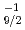
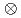
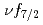 multiplet of
multiplet of  In
In
Jungclaus, A.*; Gargano, A.*; Grawe, H.*; Taprogge, J.*; Nishimura, Shunji*; Doornenbal, P.*; Lorusso, G.*; Shimizu, Y.*; Simpson, G. S.*; S derstr
derstr m, P.-A.*; et al.
m, P.-A.*; et al.
Physical Review C, 93(4), p.041301_1 - 041301_6, 2016/04
Times Cited Count:18 Percentile:74.42(Physics, Nuclear)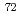 Ni
NiMorales, A. I.*; Benzoni, G.*; Watanabe, H.*; Nishimura, Shunji*; Browne, F.*; Daido, R.*; Doornenbal, P.*; Fang, Y.*; Lorusso, G.*; Patel, Z.*; et al.
Physical Review C, 93(3), p.034328_1 - 034328_14, 2016/03
Times Cited Count:26 Percentile:83.44(Physics, Nuclear) and W(CO)
and W(CO)
Usoltsev, I.*; Eichler, R.*; Wang, Y.*; Even, J.*; Yakushev, A.*; Haba, Hiromitsu*; Asai, Masato; Brand, H.*; Di Nitto, A.*; D llmann, Ch. E.*; et al.
llmann, Ch. E.*; et al.
Radiochimica Acta, 104(3), p.141 - 151, 2016/03
Times Cited Count:34 Percentile:94.43(Chemistry, Inorganic & Nuclear)Conditions of the production and decomposition of hexacarbonyl complexes of short-lived Mo and W isotopes were investigated to study thermal stability of the heaviest group 6 hexacarbonyl complex Sg(CO) . A tubular flow reactor was tested to decompose the hexacarbonyl complexes and to extract the first bond dissociation energies. A silver was found to be the most appropriate reaction surface to study the decomposition of the group 6 hexacarbonyl. It was found that the surface temperature at which the decomposition occurred was correlated to the first bond dissociation energy of Mo(CO)
. A tubular flow reactor was tested to decompose the hexacarbonyl complexes and to extract the first bond dissociation energies. A silver was found to be the most appropriate reaction surface to study the decomposition of the group 6 hexacarbonyl. It was found that the surface temperature at which the decomposition occurred was correlated to the first bond dissociation energy of Mo(CO) and W(CO)
and W(CO) , indicating that the first bond dissociation energy of Sg(CO)
, indicating that the first bond dissociation energy of Sg(CO) could be determined with this technique.
could be determined with this technique.
 decay of
decay of  Cd and excited states in
Cd and excited states in  In
InTaprogge, J.*; Jungclaus, A.*; Grawe, H.*; Nishimura, Shunji*; Doornenbal, P.*; Lorusso, G.*; Simpson, G. S.*; S derstr
derstr m, P.-A.*; Sumikama, Toshiyuki*; Xu, Z. Y.*; et al.
m, P.-A.*; Sumikama, Toshiyuki*; Xu, Z. Y.*; et al.
Physical Review C, 91(5), p.054324_1 - 054324_11, 2015/05
Times Cited Count:24 Percentile:80.08(Physics, Nuclear) -decay half-lives of 110 neutron-rich nuclei across the
-decay half-lives of 110 neutron-rich nuclei across the  =82 shell gap; Implications for the mechanism and universality of the astrophysical
=82 shell gap; Implications for the mechanism and universality of the astrophysical 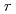 process
processLorusso, G.*; Nishimura, Shunji*; Xu, Z. Y.*; Jungclaus, A.*; Shimizu, Y.*; Simpson, G. S.*; S derstr
derstr m, P.-A.*; Watanabe, H.*; Browne, F.*; Doornenbal, P.*; et al.
m, P.-A.*; Watanabe, H.*; Browne, F.*; Doornenbal, P.*; et al.
Physical Review Letters, 114(19), p.192501_1 - 192501_7, 2015/05
Times Cited Count:174 Percentile:97.85(Physics, Multidisciplinary)Even, J.*; Ackermann, D.*; Asai, Masato; Block, M.*; Brand, H.*; Di Nitto, A.*; D llmann, Ch. E.*; Eichler, R.*; Fan, F.*; Haba, Hiromitsu*; et al.
llmann, Ch. E.*; Eichler, R.*; Fan, F.*; Haba, Hiromitsu*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(3), p.2457 - 2466, 2015/03
Times Cited Count:15 Percentile:74.22(Chemistry, Analytical)Rapid In situ synthesis of metal carbonyl complexes has been demonstrated using short-lived isotopes produced in nuclear fission and fusion reactions. The short-lived isotopes with high recoil energy directly react with carbon-monoxides and form carbonyl complexes. Only highly volatile complexes were fast transported in a gas stream to counting and chemistry devices. Short-lived Mo, Tc, Ru, Rh, W, Re, Os, and Ir were found to form volatile carbonyl complexes, while no volataile complex of Hf and Ta were detected. This technique has been applied to a chemical investigation of the superheavy element Sg (atomic number 106), and will be applicable to various fields of nuclear science with short-lived transition metal isotopes.
Rudolph, D.*; Forsberg, U.*; Golubev, P.*; Sarmiento, L. G.*; Yakushev, A.*; Andersson, L.-L.*; Di Nitto, A.*; D llmann, Ch. E.*; Gates, J. M.*; Gregorich, K. E.*; et al.
llmann, Ch. E.*; Gates, J. M.*; Gregorich, K. E.*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1185 - 1190, 2015/02
Times Cited Count:8 Percentile:52.35(Chemistry, Analytical)Thirty correlated  -decay chains of element 115 were observed, which were consistent with previous observations interpreted as the decay chain of
-decay chains of element 115 were observed, which were consistent with previous observations interpreted as the decay chain of 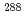 115. GEANT4 Monte-Carlo simulations were performed to reproduce high-resolution
115. GEANT4 Monte-Carlo simulations were performed to reproduce high-resolution  -photon coincidence results, which allows one to propose Q
-photon coincidence results, which allows one to propose Q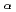 values and excitation schemes of the superheavy nuclei with unprecedented precision.
values and excitation schemes of the superheavy nuclei with unprecedented precision.
 Cd
Cd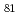 via the detection of internal conversion and Compton electrons
via the detection of internal conversion and Compton electronsTaprogge, J.*; Jungclaus, A.*; Grawe, H.*; Nishimura, Shunji*; Xu, Z. Y.*; Doornenbal, P.*; Lorusso, G.*; N cher, E.*; Simpson, G. S.*; S
cher, E.*; Simpson, G. S.*; S derstr
derstr m, P.-A.*; et al.
m, P.-A.*; et al.
Physics Letters B, 738, p.223 - 227, 2014/11
Times Cited Count:23 Percentile:78.40(Astronomy & Astrophysics) seniority isomers of
seniority isomers of 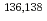 Sn
SnSimpson, G. S.*; Gey, G.*; Jungclaus, A.*; Taprogge, J.*; Nishimura, Shunji*; Sieja, K.*; Doornenbal, P.*; Lorusso, G.*; S derstr
derstr m, P.-A.*; Sumikama, Toshiyuki*; et al.
m, P.-A.*; Sumikama, Toshiyuki*; et al.
Physical Review Letters, 113(13), p.132502_1 - 132502_6, 2014/09
Times Cited Count:71 Percentile:91.52(Physics, Multidisciplinary)Even, J.*; Yakushev, A.*; D llmann, Ch. E.*; Haba, Hiromitsu*; Asai, Masato; Sato, Tetsuya; Brand, H.*; Di Nitto, A.*; Eichler, R.*; Fan, F. L.*; et al.
llmann, Ch. E.*; Haba, Hiromitsu*; Asai, Masato; Sato, Tetsuya; Brand, H.*; Di Nitto, A.*; Eichler, R.*; Fan, F. L.*; et al.
Science, 345(6203), p.1491 - 1493, 2014/09
Times Cited Count:67 Percentile:83.06(Multidisciplinary Sciences)A new superheavy element complex, a seaborgium carbonyl, has been successfully synthesized, and its adsorption property has been studied using a cryo-thermochromatography and  -detection apparatus COMPACT. Nuclear reaction products of short-lived
-detection apparatus COMPACT. Nuclear reaction products of short-lived  Sg preseparated with a gas-filled recoil ion separator GARIS at RIKEN were directly injected into a gas cell filled with He/CO mixture gas, and chemical reaction products of volatile carbonyl complexes were trasported to COMPACT. The Sg carbonyl complex detected with COMPACT was found to be very volatile with adsorption enthalpy of
Sg preseparated with a gas-filled recoil ion separator GARIS at RIKEN were directly injected into a gas cell filled with He/CO mixture gas, and chemical reaction products of volatile carbonyl complexes were trasported to COMPACT. The Sg carbonyl complex detected with COMPACT was found to be very volatile with adsorption enthalpy of 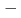 50 kJ/mol, from which we have concluded that this complex should be a Sg hexacarbonyl Sg(CO)
50 kJ/mol, from which we have concluded that this complex should be a Sg hexacarbonyl Sg(CO) . This is the first synthesis of organometallic compounds of transactinide elements for which only simple inorganic comounds have been synthesized so far.
. This is the first synthesis of organometallic compounds of transactinide elements for which only simple inorganic comounds have been synthesized so far.
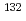 Sn explored with the long-lived isomer in
Sn explored with the long-lived isomer in  Pd
PdWatanabe, H.*; Lorusso, G.*; Nishimura, Shunji*; Otsuka, T.*; Ogawa, K.*; Xu, Z. Y.*; Sumikama, Toshiyuki*; S derstr
derstr m, P.-A.*; Doornenbal, P.*; Li, Z.*; et al.
m, P.-A.*; Doornenbal, P.*; Li, Z.*; et al.
Physical Review Letters, 113(4), p.042502_1 - 042502_6, 2014/07
Times Cited Count:25 Percentile:76.03(Physics, Multidisciplinary)