Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ostermeyer, M.*; Kong, H.-J.*; Kovalev, V. I.*; Harrison, R. G.*; Fotiadi, A. A.*; M gret, P.*; Kalal, M.*; Slezak, O.*; Yoon, J. W.*; Shin, J. S.*; et al.
gret, P.*; Kalal, M.*; Slezak, O.*; Yoon, J. W.*; Shin, J. S.*; et al.
Laser and Particle Beams, 26(3), p.297 - 362, 2008/09
Times Cited Count:41 Percentile:55.6(Physics, Applied)Kuznetsov, S. A.*; Hayashi, Hirokazu; Minato, Kazuo; Gaune-Escard, M.*
Electrochimica Acta, 51(13), p.2463 - 2470, 2006/03
Times Cited Count:90 Percentile:85.75(Electrochemistry)Electrochemical experiments were carried out at 723-823K in order to estimate separation coefficients in LiCl-KCl eutectic melt containing uranium and lanthanum trichlorides. The diffusion coefficients of U(III) were determined by electrochemical methods. The standard rate constants of charge transfer for electroreduction of uranium U(III) +3e =U were calculated by the impedance spectroscopy method. It was shown that for the calculation of uranium and lanthanum separation coefficients it is necessary to determine the voltammetric peak potentials of U(III) and La(III), their concentration in the melt and the kinetic parameters relating to U(III) discharge such as transfer and diffusion coefficients, and standard rate constants of charge transfer.
=U were calculated by the impedance spectroscopy method. It was shown that for the calculation of uranium and lanthanum separation coefficients it is necessary to determine the voltammetric peak potentials of U(III) and La(III), their concentration in the melt and the kinetic parameters relating to U(III) discharge such as transfer and diffusion coefficients, and standard rate constants of charge transfer.
Kuznetsov, S. A.*; Hayashi, Hirokazu; Minato, Kazuo; Gaune-Escard, M.*
Journal of Nuclear Materials, 344(1-3), p.169 - 172, 2005/09
Times Cited Count:26 Percentile:84.04(Materials Science, Multidisciplinary)The knowledge of separation coefficients of actinides and rare-earth metals is important for developing pyrometallurgical process of spent nuclear fuel. Electrochemical experiments were carried out at 723-823 K to estimate separation coefficients in LiCl-KCl eutectic melt containing uranium and lanthanum trichlorides. Uranium and lanthanum separation coefficients is calcurated with the voltammetric peak potentials of U (III) and La (III), their concentration in the melt and kinetic parameters for U(III) discharge such as diffusion coefficients, and standard rate constants of charge transfer. The diffusion coefficients of U (III) were determined by some electrochemical measurements. The standard rate constants of charge transfer for electroreduction of uranium U(III) +3e =U were calculated by impedance spectroscopy method.
=U were calculated by impedance spectroscopy method.
 and UCl
and UCl in a LiCl-KCl eutectic melt
in a LiCl-KCl eutectic meltKuznetsov, S. A.*; Hayashi, Hirokazu; Minato, Kazuo; Gaune-Escard, M.*
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 73(8), p.630 - 632, 2005/08
It was shown that contact of oxide materials with LiCl-KCl eutectic melt containing UCl and UCl
and UCl changes drastically the mechanism of uranium electroreduction. Transformation of voltammograms on addition of oxide ions (Li
changes drastically the mechanism of uranium electroreduction. Transformation of voltammograms on addition of oxide ions (Li O) to the melt LiCl-KCl-UCl
O) to the melt LiCl-KCl-UCl was studied. Similar changes of voltammetric curves were observed during a long contact of oxide materials with LiCl-KCl-UCl
was studied. Similar changes of voltammetric curves were observed during a long contact of oxide materials with LiCl-KCl-UCl molten system due to appearance of oxide ions in the melt resulting from oxide materials corrosion.
molten system due to appearance of oxide ions in the melt resulting from oxide materials corrosion.
 and UCl
and UCl dissolved in a LiCl-KCl eutectic melt
dissolved in a LiCl-KCl eutectic meltKuznetsov, S. A.*; Hayashi, Hirokazu; Minato, Kazuo; Gaune-Escard, M.*
Journal of the Electrochemical Society, 152(4), p.C203 - C212, 2005/04
Times Cited Count:108 Percentile:94.63(Electrochemistry)The electrochemical behavior of UCl and UCl
and UCl dissolved in LiCl-KCl eutectic melt was studied at 723-823 K. Electroreduction of U(IV) in LiCl-KCl melt occurs via two successive steps involving transfer of one and three electrons. The diffusion coefficients of U(IV) and U(III) ions were determined by linear sweep voltammetry, chronopotentiometry, and chronoamperometry. The formal standard potential of E
dissolved in LiCl-KCl eutectic melt was studied at 723-823 K. Electroreduction of U(IV) in LiCl-KCl melt occurs via two successive steps involving transfer of one and three electrons. The diffusion coefficients of U(IV) and U(III) ions were determined by linear sweep voltammetry, chronopotentiometry, and chronoamperometry. The formal standard potential of E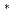 U(IV)/U(III), E
U(IV)/U(III), E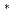 U(IV)/U, and E
U(IV)/U, and E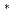 U(III)/U were determined and some thermodynamic properties of UCl
U(III)/U were determined and some thermodynamic properties of UCl and UCl
and UCl dissolved in LiCl-KCl eutectic melt were calculated. Influence of oxide ions on electrochemical behavior was also studied.
dissolved in LiCl-KCl eutectic melt were calculated. Influence of oxide ions on electrochemical behavior was also studied.