Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kyono, Atsushi*; Yamamoto, Genichiro*; Yoneda, Yasuhiro; Okada, Satoru*
Isotope News, (783), p.23 - 27, 2022/10
Mineral traps are attracting attention as an underground storage method for carbon dioxide. Carbon dioxide laden groundwater reacts with basalt to form magnesite. The formed magnesium carbonate phase varies in many ways, but we tried to clarify the structure because all of them pass through amorphous magnesium carbonate. Pair distribution function using high-energy X-ray diffraction revealed that amorphous magnesium carbonate has a structure similar to that of hydromagnesite. It can be said that it is a safe sequestration method as a carbon dioxide storage technology.

 3H(D)
3H(D) O by neutron diffraction and effect of pH on structural formulas of nesquehonite
O by neutron diffraction and effect of pH on structural formulas of nesquehoniteYamamoto, Genichiro*; Kyono, Atsushi*; Abe, Jun*; Sano, Asami; Hattori, Takanori
Journal of Mineralogical and Petrological Sciences, 116(2), p.96 - 103, 2021/04
Times Cited Count:7 Percentile:46.55(Mineralogy)Neutron diffraction, Raman spectroscopy, and thermal analysis were performed to investigate the composition, structure, and formation conditions of the magnesium carbonate hydrate nesquehonite. The time-of-flight neutron diffraction revealed the crystal structure of the monoclinic space group  2
2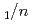 with lattice parameters of
with lattice parameters of 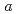 =7.72100(12)
=7.72100(12)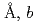 =5.37518(7)
=5.37518(7)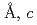 =12.1430(3)
=12.1430(3)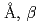 =90.165(4)
=90.165(4) , in which two deuterium atoms are coordinated to the O1, O2, and O6 atoms to form water molecules. The three water molecules in the structure suggests the structural formula of the nesquehonite should be MgCO
, in which two deuterium atoms are coordinated to the O1, O2, and O6 atoms to form water molecules. The three water molecules in the structure suggests the structural formula of the nesquehonite should be MgCO
 3H
3H O rather than Mg(HCO
O rather than Mg(HCO )(OH)
)(OH)  2H
2H O.
O.
 Al
Al (O
(O D
D )
) , with temperature at high pressure
, with temperature at high pressureKyono, Atsushi*; Kato, Masato*; Sano, Asami; Machida, Shinichi*; Hattori, Takanori
Physics and Chemistry of Minerals, 46(5), p.459 - 469, 2019/05
Times Cited Count:4 Percentile:16.46(Materials Science, Multidisciplinary)To reveal the decomposition mechanism with temperature under high-pressure, crystal structure of a hydrogrossular, katoite Ca Al
Al (O
(O D
D )
) has been studied by in-situ neutron diffraction at 8 GPa. Although unusual expansion behavior was discerned at 200-400
has been studied by in-situ neutron diffraction at 8 GPa. Although unusual expansion behavior was discerned at 200-400 C, the unit cell was continuously expanded up to 850
C, the unit cell was continuously expanded up to 850 C. At 900
C. At 900 C, katoite was decomposed, indicating that pressure strongly increases dehydration temperature from 300
C, katoite was decomposed, indicating that pressure strongly increases dehydration temperature from 300 C to 900
C to 900 C. On release of pressure, the katoite reappear together with corundum and portlandite. At 8 GPa, CaO
C. On release of pressure, the katoite reappear together with corundum and portlandite. At 8 GPa, CaO and AlO
and AlO polyhedra expand with temperature up to 850
polyhedra expand with temperature up to 850 C by about 8% and 13%, respectively. On the other hand, tetrahedral interstices are isotopically squeezed by about 10%: due to the expansion of above polyhedra. The neighboring D-D distance remains almost unchanged in this temperature range, while the O-D bond distance shrinks drastically just before decomposition. This finding suggests that the shortening of O-D distance caused by the D-D repulsion destabilizes the O-D bond, which induces the thermal decomposition of katoite.
C by about 8% and 13%, respectively. On the other hand, tetrahedral interstices are isotopically squeezed by about 10%: due to the expansion of above polyhedra. The neighboring D-D distance remains almost unchanged in this temperature range, while the O-D bond distance shrinks drastically just before decomposition. This finding suggests that the shortening of O-D distance caused by the D-D repulsion destabilizes the O-D bond, which induces the thermal decomposition of katoite.