Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Xu, J.*; Lang, P.*; Liang, S.*; Zhang, J.*; Fei, Y.*; Wang, Y.*; Gao, D.*; Hattori, Takanori; Abe, Jun*; Dong, X.*; et al.
Journal of Physical Chemistry Letters (Internet), p.2445 - 2451, 2025/00
Times Cited Count:0 Percentile:0.00(Chemistry, Physical)The Alder-ene reaction is a chemical reaction between an alkene with an allylic hydrogen, and it provides an efficient method to construct the C-C bond. Traditionally, this reaction requires catalysts, high temperatures, or photocatalysis. In this study, we reported a high-pressure-induced solid-state Alder-ene reaction of 1-hexene at room temperature without a catalyst. 1-Hexene crystallizes at 4.3 GPa and polymerizes at 18 GPa, forming olefins. By exploring gas chromatography-mass spectrometry, we discovered that 1-hexene generates dimeric products through the Alder-ene reaction under high pressures. The in situ neutron diffraction shows that the reaction process did not obey the topochemical rule. A six-membered ring transition state including one C-H  bond and two alkene
bond and two alkene  bonds was evidenced by the theoretical calculation, whose energy obviously decreased when compressed to 20 GPa. Our work offers a novel and promising method to realize the Alder-ene reaction at room temperature without a catalyst, expanding the application of this important reaction.
bonds was evidenced by the theoretical calculation, whose energy obviously decreased when compressed to 20 GPa. Our work offers a novel and promising method to realize the Alder-ene reaction at room temperature without a catalyst, expanding the application of this important reaction.
Liu, P.-F.*; Li, X.*; Li, J.*; Zhu, J.*; Tong, Z.*; Kofu, Maiko*; Nirei, Masami; Xu, J.*; Yin, W.*; Wang, F.*; et al.
National Science Review, 11(12), p.nwae216_1 - nwae216_10, 2024/12
Times Cited Count:13 Percentile:94.32(Multidisciplinary Sciences) Be ground-state molecular structure using
Be ground-state molecular structure using  Be(
Be(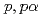 )
) He triple differential reaction cross-section measurements
He triple differential reaction cross-section measurementsLi, P. J.*; Beaumel, D.*; Lee, J.*; Assi , M.*; Chen, S.*; Franchoo, S.*; Gibelin, J.*; Hammache, F.*; Harada, T.*; Kanada-En'yo, Yoshiko*; et al.
, M.*; Chen, S.*; Franchoo, S.*; Gibelin, J.*; Hammache, F.*; Harada, T.*; Kanada-En'yo, Yoshiko*; et al.
Physical Review Letters, 131(21), p.212501_1 - 212501_7, 2023/11
Times Cited Count:21 Percentile:94.59(Physics, Multidisciplinary)The cluster structure of the neutron-rich isotope  Be has been probed via the (
Be has been probed via the (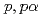 ) reaction. The triple differential cross-section was extracted and compared to distorted-wave impulse approximation reaction calculations performed in a microscopic framework using the Tohsaki-Horiuchi-Schuck-R
) reaction. The triple differential cross-section was extracted and compared to distorted-wave impulse approximation reaction calculations performed in a microscopic framework using the Tohsaki-Horiuchi-Schuck-R pke wave function and the wave function deduced from Antisymmetrized Molecular Dynamics calculations. The remarkable agreement between calculated and measured cross-sections in both shape and magnitude validates the description of the
pke wave function and the wave function deduced from Antisymmetrized Molecular Dynamics calculations. The remarkable agreement between calculated and measured cross-sections in both shape and magnitude validates the description of the  Be ground-state as a rather compact nuclear molecule.
Be ground-state as a rather compact nuclear molecule.
 100 MeV/nucleon
100 MeV/nucleonPohl, T.*; Sun, Y. L.*; Obertelli, A.*; Lee, J.*; G mez-Ramos, M.*; Ogata, Kazuyuki*; Yoshida, Kazuki; Cai, B. S.*; Yuan, C. X.*; Brown, B. A.*; et al.
mez-Ramos, M.*; Ogata, Kazuyuki*; Yoshida, Kazuki; Cai, B. S.*; Yuan, C. X.*; Brown, B. A.*; et al.
Physical Review Letters, 130(17), p.172501_1 - 172501_8, 2023/04
Times Cited Count:13 Percentile:87.42(Physics, Multidisciplinary)We report on the first proton-induced single proton- and neutron-removal reactions from the neutron deficient  O nucleus with large Fermi-surface asymmetry at
O nucleus with large Fermi-surface asymmetry at  100 MeV/nucleon. Our results provide the first quantitative contributions of multiple reaction mechanisms including the quasifree knockout, inelastic scattering, and nucleon transfer processes. It is shown that the inelastic scattering and nucleon transfer, usually neglected at such energy regime, contribute about 50% and 30% to the loosely bound proton and deeply bound neutron removal, respectively.
100 MeV/nucleon. Our results provide the first quantitative contributions of multiple reaction mechanisms including the quasifree knockout, inelastic scattering, and nucleon transfer processes. It is shown that the inelastic scattering and nucleon transfer, usually neglected at such energy regime, contribute about 50% and 30% to the loosely bound proton and deeply bound neutron removal, respectively.
Zhang, J.*; Kuang, L.*; Mou, Z.*; Kondo, Toshiaki*; Koarashi, Jun; Atarashi-Andoh, Mariko; Li, Y.*; Tang, X.*; Wang, Y.-P.*; Pe uelas, J.*; et al.
uelas, J.*; et al.
Plant and Soil, 481(1-2), p.349 - 365, 2022/12
Times Cited Count:12 Percentile:71.66(Agronomy) W and
W and 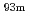 Mo as lighter homologues of seaborgium (Sg) in HF/HNO
Mo as lighter homologues of seaborgium (Sg) in HF/HNO on anion-exchange resin
on anion-exchange resinLiang, X. H.*; Tsukada, Kazuaki; Toyoshima, Atsushi; Li, Z.*; Asai, Masato; Sato, Tetsuya; Sato, Nozomi; Nagame, Yuichiro
Journal of Radioanalytical and Nuclear Chemistry, 292(2), p.917 - 922, 2012/05
Times Cited Count:9 Percentile:54.18(Chemistry, Analytical)Adsorption of carrier-free radiotracers  W and
W and 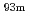 Mo produced in the
Mo produced in the  Ta(
Ta( ,
,  ) and
) and  Nb(
Nb( ,
,  ) reactions, respectively, on anion-exchange resin was studied in mixed solution of HF and HNO
) reactions, respectively, on anion-exchange resin was studied in mixed solution of HF and HNO in a concentration range of 10
in a concentration range of 10 - 10
- 10 M HF/0.1 M HNO
M HF/0.1 M HNO . Distribution coefficients (
. Distribution coefficients ( ) of
) of  W and
W and 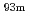 Mo at 70
Mo at 70  C showed the V-shaped variation with the minimum at around 10
C showed the V-shaped variation with the minimum at around 10 M HF/0.1 M HNO
M HF/0.1 M HNO although variation of the
although variation of the  values for
values for 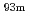 Mo was quite small compared with that for
Mo was quite small compared with that for  W. Formation of oxofluoro complexes for W and Mo is briefly discussed.
W. Formation of oxofluoro complexes for W and Mo is briefly discussed.
 SO
SO /HNO
/HNO mixed solution
mixed solutionLi, Z.*; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Kikuchi, Takahiro; Nagame, Yuichiro; Sch del, M.; Pershina, V.*; et al.
del, M.; Pershina, V.*; et al.
Radiochimica Acta, 100(3), p.157 - 164, 2012/03
Times Cited Count:14 Percentile:68.81(Chemistry, Inorganic & Nuclear) SO
SO /HNO
/HNO mixed solution ([H
mixed solution ([H ] = 1.0 M)
] = 1.0 M)Li, Z.; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Kikuchi, Takahiro; Sch del, M.*; Nagame, Yuichiro; Liang, X. H.*; et al.
del, M.*; Nagame, Yuichiro; Liang, X. H.*; et al.
no journal, ,
Cation-exchange behavior of  Rf (T
Rf (T = 78 s) produced in the
= 78 s) produced in the  Cm(
Cm( O, 5n) reaction was studied on a "one-atom-at-a-time" scale in H
O, 5n) reaction was studied on a "one-atom-at-a-time" scale in H SO
SO (0.15-0.69 M)/HNO
(0.15-0.69 M)/HNO mixed solutions ([H
mixed solutions ([H ] = 1.0 M) using an automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy. It was found that adsorption probability (%ads) of
] = 1.0 M) using an automated ion-exchange separation apparatus coupled with the detection system for alpha-spectroscopy. It was found that adsorption probability (%ads) of  Rf on cation-exchange resin decreases with increasing [HSO
Rf on cation-exchange resin decreases with increasing [HSO
 ], showing a successive formation of its sulfate complexes. Rf exhibited much weaker formation of the complexes than the lighter homologues Zr and Hf, which is qualitatively in good agreement with theoretical calculations including relativistic effects.
], showing a successive formation of its sulfate complexes. Rf exhibited much weaker formation of the complexes than the lighter homologues Zr and Hf, which is qualitatively in good agreement with theoretical calculations including relativistic effects.
 SO
SO /HNO
/HNO mixed solution ([H
mixed solution ([H ] = 1.0 M)
] = 1.0 M)Li, Z.; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Sch del, M.*; Nagame, Yuichiro; Liang, X. H.*; Kasamatsu, Yoshitaka*; et al.
del, M.*; Nagame, Yuichiro; Liang, X. H.*; Kasamatsu, Yoshitaka*; et al.
no journal, ,
Cation-exchange behavior of  Rf (T
Rf (T = 78 s) produced in the
= 78 s) produced in the  Cm(
Cm( O,5n) reaction was studied on a "one-atom-at-a-time" scale in 0.15-0.69 M H
O,5n) reaction was studied on a "one-atom-at-a-time" scale in 0.15-0.69 M H SO
SO /HNO
/HNO mixed solutions ([H
mixed solutions ([H ] = 1.0 M) using an automated ion-exchange separation apparatus coupled with a detection system for alpha-spectroscopy (AIDA). It was found that adsorption probabilities (%ads) of Rf on the cation-exchange resin decrease with increasing [HSO
] = 1.0 M) using an automated ion-exchange separation apparatus coupled with a detection system for alpha-spectroscopy (AIDA). It was found that adsorption probabilities (%ads) of Rf on the cation-exchange resin decrease with increasing [HSO
 ], showing successive formation of Rf sulfate complexes. Rf exhibits a weaker complex formation than the lighter homologues Zr and Hf.
], showing successive formation of Rf sulfate complexes. Rf exhibits a weaker complex formation than the lighter homologues Zr and Hf.
Sato, Tetsuya; Tsukada, Kazuaki; Asai, Masato; Toyoshima, Atsushi; Kasamatsu, Yoshitaka*; Li, Z.; Sato, Nozomi; Kikuchi, Takahiro; Liang, X. H.*; Kaneya, Yusuke*; et al.
no journal, ,
To investigate chemical properties of the superheavy element dubnium (Db, Z = 105), we have so far developed an on-line isothermal gas chromatographic apparatus. In this work, the on-line experiments with group-5 elements, Nb and Ta, using short-lived isotopes 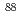 Nb and
Nb and 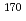 Ta as the homologues of Db were carried out. We determined optimum conditions to produce volatile compounds of the elements. Under the obtained conditions, overall efficiencies of the volatile compounds were measured as a function of the isothermal temperature in chlorinating condition with mixture of nitrogen gas and the SOCl
Ta as the homologues of Db were carried out. We determined optimum conditions to produce volatile compounds of the elements. Under the obtained conditions, overall efficiencies of the volatile compounds were measured as a function of the isothermal temperature in chlorinating condition with mixture of nitrogen gas and the SOCl vapor containing 1% oxygen. Based on the isothermal gas chromatographic behavior of them, it was found that Ta compounds would be less volatile than Nb ones.
vapor containing 1% oxygen. Based on the isothermal gas chromatographic behavior of them, it was found that Ta compounds would be less volatile than Nb ones.