Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tanaka, Kazuya; Yamaji, Keiko*; Masuya, Hayato*; Tomita, Jumpei; Ozawa, Mayumi*; Yamasaki, Shinya*; Tokunaga, Kohei; Fukuyama, Kenjin*; Ohara, Yoshiyuki*; Maamoun, I.*; et al.
Chemosphere, 355, p.141837_1 - 141837_11, 2024/05
In this study, biogenic Mn(IV) oxide was applied to remove Ra from mine water collected from a U mill tailings pond in the Ningyo-toge center. Just 7.6 mg of biogenic Mn(IV) oxide removed more than 98% of the  Ra from 3 L of mine water, corresponding to a distribution coefficient of 10
Ra from 3 L of mine water, corresponding to a distribution coefficient of 10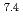 mL/g for Ra at pH 7. The obtained value was convincingly high for practical application of biogenic Mn(IV) oxide in water treatment.
mL/g for Ra at pH 7. The obtained value was convincingly high for practical application of biogenic Mn(IV) oxide in water treatment.
Maamoun, I.; Falyouna, O.*; Shariful, I. M.*; Eljamal, R.*; Bensaida, K.*; Tanaka, Kazuya; Tokunaga, Kohei; Eljamal, O.*
Advanced Materials Letters (Internet), 14(2), p.23021721_1 - 23021721_10, 2023/04
 bimetallic Ni/Fe
bimetallic Ni/Fe nanoparticles; Implications towards Tc(VII) removal
nanoparticles; Implications towards Tc(VII) removalMaamoun, I.; Tokunaga, Kohei; Dohi, Terumi; Kanno, Futoshi*; Falyouna, O.*; Eljamal, O.*; Tanaka, Kazuya
Frontiers in Nuclear Engineering (Internet), 2, p.1142823_1 - 1142823_17, 2023/03
Recently, the rapid development of nuclear power technologies and the continuous energy demand around the world exhibited massive amounts of contaminated water with radionuclides. Technetium-99 ( Tc) is one of the long-lived radionuclides with a highly mobile anionic form (Tc(VII)) in aqueous solutions. Hence, the rapid and efficient Tc(VII) removal from radioactive water has emerged as a challenging issue. In this work, bimetallic nickel/iron nanoparticles (Ni/Fe
Tc) is one of the long-lived radionuclides with a highly mobile anionic form (Tc(VII)) in aqueous solutions. Hence, the rapid and efficient Tc(VII) removal from radioactive water has emerged as a challenging issue. In this work, bimetallic nickel/iron nanoparticles (Ni/Fe ) were prepared to enhance the immobilization of rhenium (Re(VII)) from aqueous solutions, as the surrogate of Tc(VII).
) were prepared to enhance the immobilization of rhenium (Re(VII)) from aqueous solutions, as the surrogate of Tc(VII).
Maamoun, I.; Rushdi, M.*; Falyouna, O.*; Eljamal, R.*; Eljamal, O.*
Separation and Purification Technology, 308, p.122863_1 - 122863_16, 2023/03
Times Cited Count:10 Percentile:44.82(Engineering, Chemical) @Mg(OH)
@Mg(OH) ) into porous media for Cr(VI) removal from groundwater
) into porous media for Cr(VI) removal from groundwaterMaamoun, I.; Falyouna, O.*; Eljamal, R.*; Idham, M. F.*; Tanaka, Kazuya; Eljamal, O.*
Chemical Engineering Journal, 451, Part3, p.138718_1 - 138718_22, 2023/01
Times Cited Count:46 Percentile:92.38(Engineering, Environmental)
 removal from aqueous solutions; Cost-effectiveness & parametric effects
removal from aqueous solutions; Cost-effectiveness & parametric effectsMaamoun, I.; Eljamal, R.*; Eljamal, O.*
Chemosphere, 312, Part 1, p.137176_1 - 137176_11, 2023/01
Times Cited Count:17 Percentile:78.09(Environmental Sciences) and nano zero-valent iron
and nano zero-valent ironIslam, M. S.*; Maamoun, I.; Falyouna, O.*; Eljamal, O.*; Saha, B. B.*
Journal of Molecular Liquids, 370, p.121005_1 - 121005_11, 2023/01
Times Cited Count:43 Percentile:98.27(Chemistry, Physical)Falyouna, O.*; Maamoun, I.; Ghosh, S.*; Malloum, A.*; Othmani, A.*; Eljamal, O.*; Amen, T. W. M.*; Oroke, A.*; Bornman, C.*; Ahmadi, S.*; et al.
Journal of Molecular Liquids, 368, Part B, p.120726_1 - 120726_25, 2022/12
Times Cited Count:15 Percentile:48.95(Chemistry, Physical)Idham, M. F.*; Falyouna, O.*; Eljamal, R.*; Maamoun, I.; Eljamal, O.*
Journal of Water Process Engineering (Internet), 50, p.103289_1 - 103289_16, 2022/12
Times Cited Count:36 Percentile:95.24(Engineering, Environmental)Singha, B.*; Eljamal, O.*; Karmaker, S. C.*; Maamoun, I.; Sugihara, Yuji*
Journal of Environmental Management, 317, p.115484_1 - 115484_9, 2022/09
Times Cited Count:28 Percentile:87.81(Environmental Sciences)Khalil, A. M. E.*; Han, L.*; Maamoun, I.; Tabish, T. A.*; Chen, Y.*; Eljamal, O.*; Zhang, S.*; Butler, D.*; Memon, F. A.*
Advanced Sustainable Systems (Internet), 6(8), p.2200016_1 - 2200016_16, 2022/08
Times Cited Count:8 Percentile:44.71(Green & Sustainable Science & Technology)Falyouna, O.*; Idham, M. F.*; Maamoun, I.; Bensaida, K.*; Ashik, U. P. M.*; Sugihara, Yuji*; Eljamal, O.*
Journal of Molecular Liquids, 359, p.119323_1 - 119323_20, 2022/08
Times Cited Count:50 Percentile:98.89(Chemistry, Physical)Maamoun, I.; Bensaida, K.*; Eljamal, R.*; Falyouna, O.*; Tanaka, Kazuya; Tosco, T.*; Sugihara, Yuji*; Eljamal, O.*
Journal of Molecular Liquids, 358, p.119216_1 - 119216_13, 2022/07
Times Cited Count:40 Percentile:98.15(Chemistry, Physical)Maamoun, I.; Falyouna, O.*; Eljamal, R.*; Bensaida, K.*; Tanaka, Kazuya; Tosco, T.*; Sugihara, Yuji*; Eljamal, O.*
Journal of Environmental Chemical Engineering, 10(3), p.107431_1 - 107431_17, 2022/06
Times Cited Count:47 Percentile:93.04(Engineering, Environmental)Eljamal, O.*; Maamoun, I.; Alkhudhayri, S.*; Eljamal, R.*; Falyouna, O.*; Tanaka, Kazuya; Kozai, Naofumi; Sugihara, Yuji*
Journal of Water Process Engineering (Internet), 46, p.102608_1 - 102608_13, 2022/04
Times Cited Count:48 Percentile:97.39(Engineering, Environmental)Eljamal, R.*; Maamoun, I.; Bensaida, K.*; Yilmaz, G.*; Sugihara, Yuji*; Eljamal, O.*
Renewable and Sustainable Energy Reviews, 158, p.112192_1 - 112192_13, 2022/04
Times Cited Count:41 Percentile:93.64(Green & Sustainable Science & Technology) /MgO] for prompt and efficient adsorption of ciprofloxacin from aqueous solutions
/MgO] for prompt and efficient adsorption of ciprofloxacin from aqueous solutionsFalyouna, O.*; Bensaida, K.*; Maamoun, I.; Ashik, U. P. M.*; Tahara, Atsushi*; Tanaka, Kazuya; Aoyagi, Noboru; Sugihara, Yuji*; Eljamal, O.*
Journal of Cleaner Production, 342, p.130949_1 - 130949_15, 2022/03
Times Cited Count:64 Percentile:98.03(Green & Sustainable Science & Technology)Bensaida, K.*; Maamoun, I.; Eljamal, R.*; Falyouna, O.*; Sugihara, Yuji*; Eljamal, O.*
Energy Conversion and Management, 249, p.114877_1 - 114877_12, 2021/12
Times Cited Count:44 Percentile:93.62(Thermodynamics)Maamoun, I.; Falyouna, O.*; Shariful, I. M.*; Eljamal, R.*; Bensaida, K.*; Tanaka, Kazuya; Tokunaga, Kohei; Eljamal, O.*
no journal, ,
Tanaka, Kazuya; Kurihara, Yuichi*; Tomita, Jumpei; Maamoun, I.; Yamasaki, Shinya*; Tokunaga, Kohei; Fukuyama, Kenjin*; Kozai, Naofumi
no journal, ,
We collected surface sediment and water samples at a U mill tailings pond in the Ningyo-toge center. Radioactivity concentrations of  Ra in sediments were 12,000 - 29,000 Bq/kg while those in water ranged from 60 to 580 mBq/L. As a result, apparent distribution coefficients of Ra between ferric sediment and water were estimated to be 3.1
Ra in sediments were 12,000 - 29,000 Bq/kg while those in water ranged from 60 to 580 mBq/L. As a result, apparent distribution coefficients of Ra between ferric sediment and water were estimated to be 3.1  10
10 - 3.8
- 3.8  10
10 mL/g, which were higher than reported ones for ferrihydrite and goethite. This suggests that another host on which Ra is strongly fixed would be present in sediment if we assume equilibrium between sediment and water. It is possible that Mn(IV) oxide as a minor component contributed to an increase in the apparent distribution coefficients. Another possibility is upward flow of pore water containing
mL/g, which were higher than reported ones for ferrihydrite and goethite. This suggests that another host on which Ra is strongly fixed would be present in sediment if we assume equilibrium between sediment and water. It is possible that Mn(IV) oxide as a minor component contributed to an increase in the apparent distribution coefficients. Another possibility is upward flow of pore water containing  Ra or diffusion of
Ra or diffusion of  Ra released from lower sediment layers. Either scenario provides additional input of
Ra released from lower sediment layers. Either scenario provides additional input of  Ra to surface sediment. In this case, surface sediment and water did not reach equilibrium with each other, but
Ra to surface sediment. In this case, surface sediment and water did not reach equilibrium with each other, but  Ra concentrations in surface sediments were governed by dynamics through input of
Ra concentrations in surface sediments were governed by dynamics through input of  Ra from porewater as well as groundwater in sediment-water interaction.
Ra from porewater as well as groundwater in sediment-water interaction.