Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Machida, Akihiko*; Saito, Hiroyuki*; Aoki, Katsutoshi*; Komatsu, Kazuki*; Hattori, Takanori; Sano, Asami; Funakoshi, Kenichi*; Machida, Shinichi*; Sato, Toyoto*; Orimo, Shinichi*
Physical Review B, 111(22), p.224413_1 - 224413_6, 2025/06
The crystal and magnetic structures of antiferromagnetic Mn deuterides formed by hydrogenating Mn metal at high temperature and high pressure, fcc  -MnDx and hcp
-MnDx and hcp 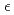 -MnDx, were investigated by in-situ neutron powder diffraction. Deuterium atoms partially occupied the octahedral interstitial positions of the fcc and hcp metal lattices. The site occupancies increased rapidly with decreasing temperature from
-MnDx, were investigated by in-situ neutron powder diffraction. Deuterium atoms partially occupied the octahedral interstitial positions of the fcc and hcp metal lattices. The site occupancies increased rapidly with decreasing temperature from  700 to
700 to  450 K and remained down to 300 K. N
450 K and remained down to 300 K. N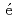 el temperature of 543(10) K was determined for
el temperature of 543(10) K was determined for  -MnD
-MnD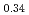 . For
. For 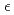 -MnD
-MnD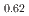 , saturation magnetic moment and N
, saturation magnetic moment and N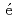 el temperature were determined to be 0.82(1)
el temperature were determined to be 0.82(1) 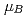 and 347(3) K, respectively. The N
and 347(3) K, respectively. The N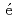 el temperatures determined for
el temperatures determined for  -MnD
-MnD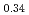 and
and 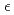 -MnD
-MnD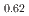 are consistent with those predicted by the respective Slater-Pauling curves proposed in previous studies. The updated N
are consistent with those predicted by the respective Slater-Pauling curves proposed in previous studies. The updated N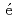 el temperatures provide insights into the development of more accurate Slater-Pauling curves based on electronic band structure calculations.
el temperatures provide insights into the development of more accurate Slater-Pauling curves based on electronic band structure calculations.
Aoki, Katsutoshi*; Machida, Akihiko*; Saito, Hiroyuki*; Hattori, Takanori
Koatsuryoku No Kagaku To Gijutsu, 35(1), p.4 - 11, 2025/03
Iron reacts with hydrogen to form solid solutions with body-centered cubic, face-centered cubic, hexagonal close packed, and double hexagonal close packed structures at high temperatures and high pressures. Neutron diffraction is the most powerful tool for determining the occupation sites and occupancies of hydrogen atoms dissolved in a metal lattice. Structural parameters, including hydrogen occupation sites and occupancies, are refined via Rietveld analysis for neutron diffraction data. We present our expertise in Rietveld refinement of iron hydrides accumulated over 10 years.
Im, S.*; Jee, H.*; Kanematsu, Manabu*; Morooka, Satoshi; Choe, H.*; Nishio, Yuhei*; Machida, Akihiko*; Tominaga, Aki; Jeon, B. H.*; Bae, S.*
Construction and Building Materials, 459, p.139742_1 - 139742_17, 2025/01
Times Cited Count:0 Percentile:0.00(Construction & Building Technology)Machida, Akihiko*; Saito, Hiroyuki*; Sugimoto, Hidehiko*; Hattori, Takanori; Sano, Asami; Endo, Naruki*; Katayama, Yoshinori*; Iizuka, Riko*; Sato, Toyoto*; Matsuo, Motoaki*; et al.
Nature Communications (Internet), 15, p.8861_1 - 8861_2, 2024/10
Times Cited Count:0 Percentile:0.00(Multidisciplinary Sciences)In our previous article (Nature Commun. 5, 5063 (2014)), the site occupancies of D atoms dissolved in an fcc Fe metal lattice were investigated via Rietveld refinement of neutron powder diffraction patterns collected at 988 K and 6.3 GPa. The fcc metal lattice has two interstitial sites available for accommodating D atoms: octahedral and tetrahedral sites. The Rietveld refinement revealed that D atoms occupied mainly the octahedral sites with occupancy of 0.532 and slightly the tetrahedral sites with occupancy of 0.056. Subsequent density-functional-theory (DFT) calculations by Antonov (Phys. Rev. Mater. 2019)) showed that the occupation energy on the tetrahedral site was significantly higher than that on the octahedral site; the tetrahedral site occupation was unlikely to occur even at temperatures as high as 988 K. We reexamined the site occupancies of D-atom by Rietveld refinement including extinction correction. As a result, the octahedral occupancy was increased to 0.60 and the tetrahedral occupancy was reduced to zero. The occupation of only the octahedral site for D atom is consistent with the DFT calculation, although in contrast to the previous results.
Kim, G.*; Cho, S.-M.*; Im, S.*; Suh, H.*; Morooka, Satoshi; Shobu, Takahisa; Kanematsu, Manabu*; Machida, Akihiko*; Bae, S.*
Construction and Building Materials, 411, p.134529_1 - 134529_18, 2024/01
Times Cited Count:8 Percentile:69.96(Construction & Building Technology)Ikeda, Kazutaka*; Sashida, Sho*; Otomo, Toshiya*; Oshita, Hidetoshi*; Honda, Takashi*; Hawai, Takafumi*; Saito, Hiraku*; Ito, Shinichi*; Yokoo, Tetsuya*; Sakaki, Koji*; et al.
International Journal of Hydrogen Energy, 51(Part A), p.79 - 87, 2024/01
Times Cited Count:6 Percentile:48.53(Chemistry, Physical)Cho, S.*; Suh, H.*; Im, S.*; Kim, G.*; Kanematsu, Manabu*; Morooka, Satoshi; Machida, Akihiko*; Shobu, Takahisa; Bae, S.*
Construction and Building Materials, 409, p.133866_1 - 133866_20, 2023/12
Times Cited Count:13 Percentile:85.36(Construction & Building Technology) synchrotron X-ray scattering and nanoindentation test
synchrotron X-ray scattering and nanoindentation testIm, S.*; Jee, H.*; Suh, H.*; Kanematsu, Manabu*; Morooka, Satoshi; Choe, H.*; Nishio, Yuhei*; Machida, Akihiko*; Kim, J.*; Lim, S.*; et al.
Construction and Building Materials, 365, p.130034_1 - 130034_18, 2023/02
Times Cited Count:17 Percentile:77.01(Construction & Building Technology)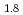 and TiH
and TiH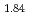 up to 21 GPa by incoherent inelastic neutron scattering
up to 21 GPa by incoherent inelastic neutron scatteringHattori, Takanori; Nakamura, Mitsutaka; Iida, Kazuki*; Machida, Akihiko*; Sano, Asami; Machida, Shinichi*; Arima, Hiroshi*; Oshita, Hidetoshi*; Honda, Takashi*; Ikeda, Kazutaka*; et al.
Physical Review B, 106(13), p.134309_1 - 134309_9, 2022/10
Times Cited Count:1 Percentile:7.32(Materials Science, Multidisciplinary)Hydrogen vibration excitations of fluorite-type ZrH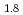 and TiH
and TiH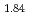 were investigated up to 21 GPa and 4 GPa, respectively, by incoherent inelastic neutron scattering experiments. The first excitation energies increased with pressure, as described by the equations
were investigated up to 21 GPa and 4 GPa, respectively, by incoherent inelastic neutron scattering experiments. The first excitation energies increased with pressure, as described by the equations 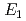 (meV) = 141.4(2) + 1.02(2)
(meV) = 141.4(2) + 1.02(2) (GPa) and
(GPa) and 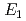 (meV) = 149.4(1) + 1.21(8)
(meV) = 149.4(1) + 1.21(8) (GPa) for ZrH
(GPa) for ZrH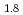 and TiH
and TiH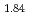 , respectively. Coupling with pressure dependence of lattice parameters, the relations between metal-hydrogen distance (
, respectively. Coupling with pressure dependence of lattice parameters, the relations between metal-hydrogen distance (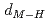 ) and
) and 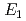 are found to be well described by the equations
are found to be well described by the equations 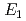 (meV) = 1.62(9)
(meV) = 1.62(9) 10
10
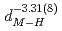 (
(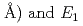 (meV) = 1.47(21)
(meV) = 1.47(21) 10
10
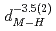 (AA), respectively. The slopes of these curves are much steep compared to the previously reported trend in various fluorite-type metal hydrides at ambient pressure. The hydrogen wave function spreading showed that the local potential field for a hydrogen atom shrinks more intensively than the tetrahedral site. These behavior is likely caused by the rigid metal ion core and the resulting confinement of the hydrogen atom in the narrower potential field at high pressures.
(AA), respectively. The slopes of these curves are much steep compared to the previously reported trend in various fluorite-type metal hydrides at ambient pressure. The hydrogen wave function spreading showed that the local potential field for a hydrogen atom shrinks more intensively than the tetrahedral site. These behavior is likely caused by the rigid metal ion core and the resulting confinement of the hydrogen atom in the narrower potential field at high pressures.
Kim, G.*; Im, S.*; Jee, H.*; Suh, H.*; Cho, S.*; Kanematsu, Manabu*; Morooka, Satoshi; Koyama, Taku*; Nishio, Yuhei*; Machida, Akihiko*; et al.
Cement and Concrete Research, 159, p.106869_1 - 106869_17, 2022/09
Times Cited Count:27 Percentile:86.85(Construction & Building Technology)Sakaki, Koji*; Kim, H.*; Majzoub, E. H.*; Machida, Akihiko*; Watanuki, Tetsu*; Ikeda, Kazutaka*; Otomo, Toshiya*; Mizuno, Masataka*; Matsumura, Daiju; Nakamura, Yumiko*
Acta Materialia, 234, p.118055_1 - 118055_10, 2022/08
Times Cited Count:20 Percentile:84.24(Materials Science, Multidisciplinary)Fuchita, Tomoki*; Urata, Taisei*; Matsuyama, Tsugufumi*; Murakami, Masashi; Yoshida, Yukihiko; Ueda, Akihiko; Machida, Masahiko; Sasaki, Toshiki; Tsuji, Koichi*
Advances in X-Ray Chemical Analysis, Japan, 53, p.77 - 87, 2022/03
X-ray fluorescence (XRF) analysis is an analytical method to obtain elemental information by detecting fluorescence X-rays emitted from a sample irradiated with X-rays. It is possible to obtain two-dimensional elemental distribution images by scanning a sample with micro X-ray beam. In this study, we developed an XRF analytical instrument to rapidly obtain the elemental distributions for moving samples on a belt conveyor by applying the micro XRF technique. X-rays were widely irradiated to the belt conveyor. The elemental distributions were measured by scanning an X-ray detector, crossing above the belt conveyor. A collimator was attached to the top of the detector to limit the analyzing area. Both detection limit and spatial resolutions for moving directions of the detector and the belt conveyor were evaluated. Finally, it was demonstrated that the multi-elemental imaging was possible with the developed XRF instrument.
Im, S.*; Jee, H.*; Suh, H.*; Kanematsu, Manabu*; Morooka, Satoshi; Koyama, Taku*; Nishio, Yuhei*; Machida, Akihiko*; Kim, J.*; Bae, S.*
Journal of the American Ceramic Society, 104(9), p.4803 - 4818, 2021/09
Times Cited Count:26 Percentile:82.77(Materials Science, Ceramics)Jee, H.*; Im, S.*; Kanematsu, Manabu*; Suzuki, Hiroshi; Morooka, Satoshi; Koyama, Taku*; Machida, Akihiko*; Bae, S.*
Journal of the American Ceramic Society, 103(12), p.7188 - 7201, 2020/12
Times Cited Count:21 Percentile:69.45(Materials Science, Ceramics)Saito, Hiroyuki*; Machida, Akihiko*; Hattori, Takanori; Sano, Asami; Funakoshi, Kenichi*; Sato, Toyoto*; Orimo, Shinichi*; Aoki, Katsutoshi*
Physica B; Condensed Matter, 587, p.412153_1 - 412153_6, 2020/06
Times Cited Count:5 Percentile:25.28(Physics, Condensed Matter)The site occupancy of deuterium (D) atoms in face-centered-cubic nickel (fcc Ni) was measured along a cooling path from 1073 to 300 K at an initial pressure of 3.36 GPa via in situ neutron powder diffraction. Deuterium atoms predominantly occupy the octahedral (O) sites and slightly occupy the tetrahedral (T) sites of the fcc metal lattice. The O-site occupancy increases from 0.4 to 0.85 as the temperature is lowered from 1073 to 300 K. Meanwhile, the T-site occupancy remains c.a. 0.02. The temperature-independent behavior of the T-site occupancy is unusual, and its process is not yet understood. From the linear relation between the expanded lattice volume and D content, a D-induced volume expansion of 2.09(13) 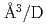 atom was obtained. This value is in agreement with the values of 2.14-2.2
atom was obtained. This value is in agreement with the values of 2.14-2.2 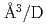 atom previously reported for Ni and Ni
atom previously reported for Ni and Ni Fe
Fe alloy.
alloy.
Saito, Hiroyuki*; Machida, Akihiko*; Iizuka, Riko*; Hattori, Takanori; Sano, Asami; Funakoshi, Kenichi*; Sato, Toyoto*; Orimo, Shinichi*; Aoki, Katsutoshi*
Scientific Reports (Internet), 10, p.9934_1 - 9934_8, 2020/06
Times Cited Count:5 Percentile:19.41(Multidisciplinary Sciences)Neutron powder diffraction profiles were collected for iron deuteride (FeDx) while the temperature decreased from 1023 to 300 K for a pressure range of 4-6 GPa. The  ' deuteride with a double hexagonal close-packed (dhcp) structure, which coexisted with other stable or metastable deutrides at each temperature and pressure condition, formed solid solutions with a composition of FeD
' deuteride with a double hexagonal close-packed (dhcp) structure, which coexisted with other stable or metastable deutrides at each temperature and pressure condition, formed solid solutions with a composition of FeD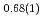 at 673 K and 6.1 GPa and FeD
at 673 K and 6.1 GPa and FeD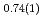 at 603 K and 4.8 GPa. Upon stepwise cooling to 300 K, the D-content x increased to a stoichiometric value of 1.0 to form monodeuteride FeD
at 603 K and 4.8 GPa. Upon stepwise cooling to 300 K, the D-content x increased to a stoichiometric value of 1.0 to form monodeuteride FeD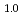 . In the dhcp FeD
. In the dhcp FeD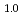 at 300 K and 4.2 GPa, dissolved D atoms fully occupied the octahedral interstitial sites, slightly displaced from the octahedral centers in the dhcp metal lattice, and the dhcp sequence of close-packed Fe planes contained hcp-stacking faults at 12%. Magnetic moments with 2.11
at 300 K and 4.2 GPa, dissolved D atoms fully occupied the octahedral interstitial sites, slightly displaced from the octahedral centers in the dhcp metal lattice, and the dhcp sequence of close-packed Fe planes contained hcp-stacking faults at 12%. Magnetic moments with 2.11  0.06 B/Fe-atom aligned ferromagnetically in parallel on the Fe planes.
0.06 B/Fe-atom aligned ferromagnetically in parallel on the Fe planes.
Bae, S.*; Jee, H.*; Suh, H.*; Kanematsu, Manabu*; Shiro, Ayumi*; Machida, Akihiko*; Watanuki, Tetsu*; Shobu, Takahisa; Morooka, Satoshi; Geng, G.*; et al.
Construction and Building Materials, 237, p.117714_1 - 117714_10, 2020/03
Times Cited Count:23 Percentile:70.41(Construction & Building Technology)Machida, Akihiko*; Saito, Hiroyuki*; Hattori, Takanori; Sano, Asami; Funakoshi, Kenichi*; Sato, Toyoto*; Orimo, Shinichi*; Aoki, Katsutoshi*
Scientific Reports (Internet), 9(1), p.12290_1 - 12290_9, 2019/08
Times Cited Count:31 Percentile:83.40(Multidisciplinary Sciences)Hexagonal close-packed iron hydride, hcp FeHx, is absent from the conventional phase diagram of the Fe-H system, although hcp metallic Fe exists stably over extensive temperature ( ) and pressure (
) and pressure ( ) conditions, including those corresponding to the Earth's inner core.
) conditions, including those corresponding to the Earth's inner core. 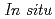 X-ray and neutron diffraction measurements at temperatures ranging from 298 to 1073 K and H pressures ranging from 4 to 7 GPa revealed that the hcp hydride was formed for FeH
X-ray and neutron diffraction measurements at temperatures ranging from 298 to 1073 K and H pressures ranging from 4 to 7 GPa revealed that the hcp hydride was formed for FeH compositions when
compositions when 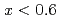 . Hydrogen atoms occupied the octahedral interstitial sites of the host metal lattice both partially and randomly. The hcp hydride exhibited a H-induced volume expansion of 2.48(5)
. Hydrogen atoms occupied the octahedral interstitial sites of the host metal lattice both partially and randomly. The hcp hydride exhibited a H-induced volume expansion of 2.48(5) 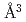 /H-atom, which was larger than that of the face-centered cubic (fcc) hydride. The hcp hydride showed an increase in
/H-atom, which was larger than that of the face-centered cubic (fcc) hydride. The hcp hydride showed an increase in  with
with  , whereas the fcc hydride showed a corresponding decrease. The present study provides guidance for further investigations of the Fe-H system over an extensive
, whereas the fcc hydride showed a corresponding decrease. The present study provides guidance for further investigations of the Fe-H system over an extensive  -
- -
- region.
region.
Bae, S.*; Jee, H.*; Kanematsu, Manabu*; Shiro, Ayumi*; Machida, Akihiko*; Watanuki, Tetsu*; Shobu, Takahisa; Suzuki, Hiroshi
Journal of the American Ceramic Society, 101(1), p.408 - 418, 2018/01
Times Cited Count:19 Percentile:61.84(Materials Science, Ceramics)Despite enormous interest in calcium silicate hydrate (C-S-H), its detailed atomic structure and intrinsic deformation under an external load are lacking. This study demonstrates the nanostructural deformation process of C-S-H in tricalcium silicate (C S) paste as a function of applied stress by interpreting atomic pair distribution function (PDF) based on in situ X-ray scattering. Three different strains in C
S) paste as a function of applied stress by interpreting atomic pair distribution function (PDF) based on in situ X-ray scattering. Three different strains in C S paste under compression were compared using a strain gauge and the real and reciprocal space PDFs. PDF refinement revealed that the C-S-H phase mostly contributed to PDF from 0 to 20
S paste under compression were compared using a strain gauge and the real and reciprocal space PDFs. PDF refinement revealed that the C-S-H phase mostly contributed to PDF from 0 to 20 whereas crystalline phases dominated that beyond 20
whereas crystalline phases dominated that beyond 20 . The short-range atomic strains exhibited two regions for C-S-H: I) plastic deformation (0-10 MPa) and II) linear elastic deformation (
. The short-range atomic strains exhibited two regions for C-S-H: I) plastic deformation (0-10 MPa) and II) linear elastic deformation ( 10 MPa), whereas the long-range deformation beyond 20
10 MPa), whereas the long-range deformation beyond 20 was similar to that of Ca(OH)
was similar to that of Ca(OH) . Below 10 MPa, the short-range strain was caused by the densification of C-S-H induced by the removal of interlayer or gel-pore water. The strain is likely to be recovered when the removed water returns to C-S-H.
. Below 10 MPa, the short-range strain was caused by the densification of C-S-H induced by the removal of interlayer or gel-pore water. The strain is likely to be recovered when the removed water returns to C-S-H.
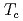 by tuning nematicity and magnetism in FeSe
by tuning nematicity and magnetism in FeSe S
S superconductors
superconductorsMatsuura, Kohei*; Mizukami, Yuta*; Arai, Yuki*; Sugimura, Yuichi*; Maejima, Naoyuki*; Machida, Akihiko*; Watanuki, Tetsu*; Fukuda, Tatsuo; Yajima, Takeshi*; Hiroi, Zenji*; et al.
Nature Communications (Internet), 8, p.1143_1 - 1143_6, 2017/10
Times Cited Count:96 Percentile:92.07(Multidisciplinary Sciences)