Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kurihara, Momo*; Yasutaka, Tetsuo*; Aono, Tatsuo*; Ashikawa, Nobuo*; Ebina, Hiroyuki*; Iijima, Takeshi*; Ishimaru, Kei*; Kanai, Ramon*; Karube, Jinichi*; Konnai, Yae*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 322(2), p.477 - 485, 2019/11
Times Cited Count:5 Percentile:33.76(Chemistry, Analytical)We assessed the repeatability and reproducibility of methods for determining low dissolved radiocesium concentrations in freshwater in Fukushima. Twenty-one laboratories pre-concentrated three of 10 L samples by five different pre-concentration methods (prussian-blue-impregnated filter cartridges, coprecipitation with ammonium phosphomolybdate, evaporation, solid-phase extraction disks, and ion-exchange resin columns), and activity of radiocesium was measured. The z-scores for all of the  Cs results were within
Cs results were within  2, indicating that the methods were accurate. The relative standard deviations (RSDs) indicating the variability in the results from different laboratories were larger than the RSDs indicating the variability in the results from each separate laboratory.
2, indicating that the methods were accurate. The relative standard deviations (RSDs) indicating the variability in the results from different laboratories were larger than the RSDs indicating the variability in the results from each separate laboratory.
Mikami, Satoshi; Maeyama, Takeshi*; Hoshide, Yoshifumi*; Sakamoto, Ryuichi*; Sato, Shoji*; Okuda, Naotoshi*; Sato, Tetsuro*; Takemiya, Hiroshi; Saito, Kimiaki
Journal of Environmental Radioactivity, 139, p.250 - 259, 2015/01
Times Cited Count:49 Percentile:78.48(Environmental Sciences)Mikami, Satoshi; Maeyama, Takeshi*; Hoshide, Yoshifumi*; Sakamoto, Ryuichi*; Sato, Shoji*; Okuda, Naotoshi*; Demongeot, S.*; Gurriaran, R.*; Uwamino, Yoshitomo*; Kato, Hiroaki*; et al.
Journal of Environmental Radioactivity, 139, p.320 - 343, 2015/01
Times Cited Count:102 Percentile:93.00(Environmental Sciences)Yamashita, Shinichi; Baldacchino, G.*; Maeyama, Takuya*; Taguchi, Mitsumasa; Muroya, Yusa*; Lin, M.*; Kimura, Atsushi; Murakami, Takeshi*; Katsumura, Yosuke
Free Radical Research, 46(7), p.861 - 871, 2012/07
Times Cited Count:26 Percentile:54.24(Biochemistry & Molecular Biology)Radiation-induced reactions in aqueous solutions of a water-soluble coumarin derivative, coumarin-3-carboxyl acid (C3CA), have been investigated by pulse radiolysis with 35-MeV electron beam, final product analysis after  Co
Co 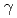 -irradiations, and deterministic model simulations. It was found that C3CA reacts with the hydroxyl radical (
-irradiations, and deterministic model simulations. It was found that C3CA reacts with the hydroxyl radical ( OH) as well as the hydrated electron at nearly diffusion-controlled rate constants: 6.8
OH) as well as the hydrated electron at nearly diffusion-controlled rate constants: 6.8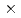 10
10 and 2.1
and 2.1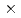 10
10 M
M s
s , respectively. Reactivity of C3CA toward O
, respectively. Reactivity of C3CA toward O

 was not confirmed. Production of a fluorescent molecule 7-hydroxy-coumarin-3-carboxylic acid (7OH-C3CA) was detected by a fluorescence spectrometer coupled with high performance liquid chromatography. Production yields of 7OH-C3CA were in a range from 0.025 to 0.18 (100 eV)
was not confirmed. Production of a fluorescent molecule 7-hydroxy-coumarin-3-carboxylic acid (7OH-C3CA) was detected by a fluorescence spectrometer coupled with high performance liquid chromatography. Production yields of 7OH-C3CA were in a range from 0.025 to 0.18 (100 eV) , depending on irradiation conditions. A variety of the yield with saturating gas, additive, and C3CA concentration implied that there are at least two pathways from scavenging reaction of C3CA toward
, depending on irradiation conditions. A variety of the yield with saturating gas, additive, and C3CA concentration implied that there are at least two pathways from scavenging reaction of C3CA toward  OH to 7OH-C3CA: peroxidation reaction followed by elimination of perhydroxyl radical and disproportionation reaction. A reaction mechanism involving the two pathways was proposed and incorporated into the simulations, showing good explanation of experimentally measured 7OH-C3CA yields with a constant conversion factor from
OH to 7OH-C3CA: peroxidation reaction followed by elimination of perhydroxyl radical and disproportionation reaction. A reaction mechanism involving the two pathways was proposed and incorporated into the simulations, showing good explanation of experimentally measured 7OH-C3CA yields with a constant conversion factor from  OH scavenging to 7OH-C3CA production, 4.7%, unless
OH scavenging to 7OH-C3CA production, 4.7%, unless 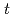 -BuOH is not added.
-BuOH is not added.
Maeyama, Takuya*; Yamashita, Shinichi; Taguchi, Mitsumasa; Baldacchino, G.*; Sihver, L.*; Murakami, Takeshi*; Katsumura, Yosuke
Radiation Physics and Chemistry, 80(12), p.1352 - 1357, 2011/12
Times Cited Count:15 Percentile:69.66(Chemistry, Physical)Coumari-3-carboxylic acid scavenges OH radical produced in water radiolysis, leading to production of a fluorescence probe at almost constant ratio relative to the amount of the scavenged OH radicals. This was applied in estimation of OH radical yield in water radiolysis especially with therapeutic heavy ions of GeV-class energies, i.e.  C
C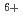 beams of 135, 290 and 400 MeV/u. OH yields upstream of the Bragg peaks decreased with increasing penetration depth of the projectile ions while that downstream suddenly jumped up to near the value for low-LET radiations such as
beams of 135, 290 and 400 MeV/u. OH yields upstream of the Bragg peaks decreased with increasing penetration depth of the projectile ions while that downstream suddenly jumped up to near the value for low-LET radiations such as  -rays. This is due to low-LET secondary fragmentation ions produced during long trajectory of the primary projectile C ion. Quantitative explanation by nuclear fragmentation simulations with PHITS code was attempted and resulted in 15-45% underestimation in the region behind the Bragg peaks, which would be due to the difference in geometries between irradiations of the sample solutions and dosimetry with a small ionization chamber.
-rays. This is due to low-LET secondary fragmentation ions produced during long trajectory of the primary projectile C ion. Quantitative explanation by nuclear fragmentation simulations with PHITS code was attempted and resulted in 15-45% underestimation in the region behind the Bragg peaks, which would be due to the difference in geometries between irradiations of the sample solutions and dosimetry with a small ionization chamber.
Maeyama, Takuya*; Yamashita, Shinichi; Baldacchino, G.*; Taguchi, Mitsumasa; Kimura, Atsushi; Murakami, Takeshi*; Katsumura, Yosuke
Radiation Physics and Chemistry, 80(4), p.535 - 539, 2011/04
Times Cited Count:41 Percentile:93.20(Chemistry, Physical)Aqueous coumarin-3-carboxylic Acid (3CCA) solutions were irradiated with eight different ion beams covering LET range from 0.5 to above 2000 eV/nm. 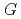 -values of 7OH-3CCA, one of hydroxylated products in radiolysis of the solutions, have been determined by fluorescence-HPLC technique in 3CCA concentration range from 0.1 to 26 mM. The formation yield of 7OH-3CCA increased with increasing concentration of 3CCA while it decreased with increasing LET value of ion beam. Compared with our previous reports on
-values of 7OH-3CCA, one of hydroxylated products in radiolysis of the solutions, have been determined by fluorescence-HPLC technique in 3CCA concentration range from 0.1 to 26 mM. The formation yield of 7OH-3CCA increased with increasing concentration of 3CCA while it decreased with increasing LET value of ion beam. Compared with our previous reports on 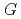 (
( OH) at a scavenging capacity of 10
OH) at a scavenging capacity of 10 s with absorption spectroscopy, it was found that
s with absorption spectroscopy, it was found that 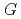 (7OH-3CCA) is about (4.7
(7OH-3CCA) is about (4.7 0.6)% of
0.6)% of 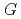 (
( OH), which is consistent for all of the ion beams used in the present study. However, 7OH-3CCA yields in high CCA concentration region, especially by using extremely high LET ions, were much higher than expected values based on the above conversion factor and
OH), which is consistent for all of the ion beams used in the present study. However, 7OH-3CCA yields in high CCA concentration region, especially by using extremely high LET ions, were much higher than expected values based on the above conversion factor and 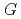 (
( OH) value predicted in theoretical work.
OH) value predicted in theoretical work.
Baldacchino, G.*; Maeyama, Takuya*; Yamashita, Shinichi; Taguchi, Mitsumasa; Kimura, Atsushi; Katsumura, Yosuke; Murakami, Takeshi*
Chemical Physics Letters, 468(4-6), p.275 - 279, 2009/01
Times Cited Count:44 Percentile:81.92(Chemistry, Physical)This paper reports a sensitive method using HPLC-fluorescence detection of  OH in liquid water under high-energy heavy-ion irradiation. The coumarin-3-carboxylic-acid (3CCA) molecule was selected for probing
OH in liquid water under high-energy heavy-ion irradiation. The coumarin-3-carboxylic-acid (3CCA) molecule was selected for probing  OH and providing the fluorescent 7-hydroxy-coumarin-3-carboxylic-acid (7OH-3CCA). Since the concentration limit achievable is better than 1 nM, the radiolytic yields were determined with a sensitivity of 2
OH and providing the fluorescent 7-hydroxy-coumarin-3-carboxylic-acid (7OH-3CCA). Since the concentration limit achievable is better than 1 nM, the radiolytic yields were determined with a sensitivity of 2 10
10 mol/J for 4.8-GeV-
mol/J for 4.8-GeV- C
C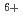 of and 20-GeV-
of and 20-GeV-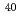 Ar
Ar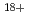 in the ns time-range. They decrease with the linear energy transfer from 2.8
in the ns time-range. They decrease with the linear energy transfer from 2.8 10
10 to 1.3
to 1.3 10
10 mol/J (
mol/J ( C
C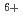 of 11 eV/nm) and 1.5
of 11 eV/nm) and 1.5 10
10 to 0.9
to 0.9 10
10 mol/J (
mol/J (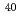 Ar
Ar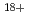 of 90 eV/nm) which is in agreement with the literature data.
of 90 eV/nm) which is in agreement with the literature data.
Yamashita, Shinichi; Katsumura, Yosuke; Lin, M.; Muroya, Yusa*; Maeyama, Takuya*; Murakami, Takeshi*
Radiation Physics and Chemistry, 77(10-12), p.1224 - 1229, 2008/10
Times Cited Count:22 Percentile:78.52(Chemistry, Physical)Measurements of primary 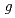 -values (at
-values (at  10
10 s after the initial ionizing event) of e
s after the initial ionizing event) of e
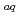 ,
,  OH and H
OH and H O
O were extended to the very high linear energy transfer (LET) region (
were extended to the very high linear energy transfer (LET) region ( 700 eV per nm) near the Bragg peak. Heavy ions (
700 eV per nm) near the Bragg peak. Heavy ions ( He
He ,
,  C
C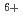 ,
, 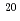 Ne
Ne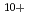 ,
, 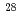 Si
Si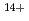 ,
, 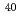 Ar
Ar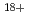 and
and 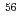 Fe
Fe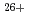 ) of energies up to 28 GeV were provided by the Heavy Ion Medical Accelerator in Chiba (HIMAC) of the National Institute of Radiological Sciences (NIRS) in Chiba, Japan. Energies of the ions were decreased down to about 10 MeV per u for
) of energies up to 28 GeV were provided by the Heavy Ion Medical Accelerator in Chiba (HIMAC) of the National Institute of Radiological Sciences (NIRS) in Chiba, Japan. Energies of the ions were decreased down to about 10 MeV per u for  He
He using an energy absorber made of polymethylmethacrylate (PMMA) plates in order to vary the LET values. Beam was visualized after passing through the energy absorber using agarose gel of aqueous solution containing methyl viologen and sodium formate in order to determine how long ions can penetrate into water. Based on the information of the penetration depth of ions in samples, much attention was paid to dose correction and LET evaluation. The obtained data were plotted as a function of (
using an energy absorber made of polymethylmethacrylate (PMMA) plates in order to vary the LET values. Beam was visualized after passing through the energy absorber using agarose gel of aqueous solution containing methyl viologen and sodium formate in order to determine how long ions can penetrate into water. Based on the information of the penetration depth of ions in samples, much attention was paid to dose correction and LET evaluation. The obtained data were plotted as a function of (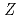
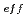 /
/ )
) also instead of LET in order to discuss effects of physical track structures on product yields, resulted in better universality.
also instead of LET in order to discuss effects of physical track structures on product yields, resulted in better universality.
Maeyama, Takuya*; Katsumura, Yosuke; Yamashita, Shinichi*; Lin, M.; Muroya, Yusa*; Miyazaki, Toyoaki*; Murakami, Takeshi*; Baldacchino, G.*
no journal, ,
no abstracts in English
Yamashita, Shinichi; Katsumura, Yosuke; Maeyama, Takuya*; Lin, M.; Muroya, Yusa*; Murakami, Takeshi*; Meesungnoen, J.*; Jay-Gerin, J.-P.*
no journal, ,
Water radiolysis with heavy ions, from helium to iron ions, of very high energies up to 28 GeV (namely several hundred MeV per nucleon) has been studied in this work. Simulations based on diffusion kinetic model were performed to examine correlation between initial track structures at the beginning of the chemical stage, 10-12 s, and primary yields as well as to estimate the validity of classical track structure model for highly energetic heavy ions.
Maeyama, Takuya*; Yamashita, Shinichi*; Baldacchino, G.*; Katsumura, Yosuke*; Taguchi, Mitsumasa; Kimura, Atsushi; Muroya, Yusa*; Murakami, Takeshi*
no journal, ,
no abstracts in English
Yamashita, Shinichi; Katsumura, Yosuke; Maeyama, Takuya*; Lin, M.; Muroya, Yusa*; Murakami, Takeshi*; Jay-Gerin, J.-P.*; Meesungnoen, J.*
no journal, ,
Although water is a main component of human cells and its interaction with ionizing radiations are of crucial importance, understanding of water radiolysis with heavy ions of energies comparable to those used in actual cancer treatments is not sufficient. Recently, such highly energetic heavy ions are available at the Heavy Ion Medical Accelerator in Chiba (HIMAC) of the National Institute of Radiological Sciences (NIRS), Chiba, Japan. We have carried out measurements of primary yields of main water decomposition products, hydrated electron (e
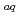 ), hydroxyl radical (
), hydroxyl radical ( OH) and hydrogen peroxide (H
OH) and hydrogen peroxide (H O
O ) with heavy ions from
) with heavy ions from  He
He to
to 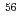 Fe
Fe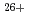 of energies up to 28 GeV (corresponding LET varies from 2 to 700 keV/
of energies up to 28 GeV (corresponding LET varies from 2 to 700 keV/ m) provided from the HIMAC. Note that primary yields are defined as the yields at 100 ns after irradiation, which can be regarded as a time scale when diffusions and intra-track reactions of initial products are almost terminated. Then, such yields are important because they inherently involve information of initial track structure and dynamics of water decomposition products during track expansion. In the present study, not only such measurements but also discussions based on the measured data were conducted by simulations.
m) provided from the HIMAC. Note that primary yields are defined as the yields at 100 ns after irradiation, which can be regarded as a time scale when diffusions and intra-track reactions of initial products are almost terminated. Then, such yields are important because they inherently involve information of initial track structure and dynamics of water decomposition products during track expansion. In the present study, not only such measurements but also discussions based on the measured data were conducted by simulations.
Yamashita, Shinichi; Katsumura, Yosuke; Maeyama, Takuya*; Lin, M.; Muroya, Yusa*; Murakami, Takeshi*; Meesungnoen, J.*; Jay-Gerin, J.-P.*
no journal, ,
It is phenomenologically well known that heavy-ion beams, which are known to be typical high-LET radiations, give distinctive irradiation effects compared to more popular low-LET radiations such as electron beam and  -rays. Here, LET is abbreviation of linear energy transfer, defined as energy deposition from radiation to surrounding matter per unit length of its trajectory. Most of earlier studies focus lighter ions (
-rays. Here, LET is abbreviation of linear energy transfer, defined as energy deposition from radiation to surrounding matter per unit length of its trajectory. Most of earlier studies focus lighter ions ( H, etc.) of lower energies (
H, etc.) of lower energies ( 10 MeV/nucleon). Moreover, knowledge under neutral pH, which is close to human body, has not been accumulated well. In this study, it is purposed to investigate chemical change occurring in neutral aqueous solutions after irradiation of high-energy (10-500 MeV/nucleon) heavy ions (
10 MeV/nucleon). Moreover, knowledge under neutral pH, which is close to human body, has not been accumulated well. In this study, it is purposed to investigate chemical change occurring in neutral aqueous solutions after irradiation of high-energy (10-500 MeV/nucleon) heavy ions ( C,
C, 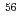 Fe, etc.). Especially, structure of heavy-ion track and radiation-chemical yields are discussed.
Fe, etc.). Especially, structure of heavy-ion track and radiation-chemical yields are discussed.
Yamashita, Shinichi; Maeyama, Takuya*; Baldacchino, G.*; Katsumura, Yosuke; Muroya, Yusa*; Taguchi, Mitsumasa; Kimura, Atsushi; Murakami, Takeshi*
no journal, ,
A novel sensitive method to determine OH yield in water radiolysis has been developed and has been applied for heavy-ion irradiations. In this work, this method was extended to measurement near the Bragg peak. It was found that OH yield has minimum value near the Bragg peak and is different even for the same ion irradiation of different acceleration energies.
Maeyama, Takuya*; Yamashita, Shinichi; Baldacchino, G.*; Katsumura, Yosuke; Muroya, Yusa*; Taguchi, Mitsumasa; Kimura, Atsushi; Murakami, Takeshi*
no journal, ,
OH yield near the Bragg peak of high-energy heavy ions, of which energies are comparable to those used in cancer therapy, has been determined in the previous work. Because fragmentations of primary projectile are significant in the energy region, it was purposed to calculate the fragmentations with an existing code and to reproduce the already obtained experimental results for verification of the code as well as for estimation of how much each fragmentated ions contributes in terms of OH yield.
Katsumura, Yosuke; Yamashita, Shinichi; Lin, M.; Maeyama, Takuya*; Muroya, Yusa*; Baldacchino, G.*; Jay-Gerin, J.-P.*; Meesungnoen, J.*; Murakami, Takeshi*
no journal, ,
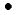 OH with therapeutic ion beam
OH with therapeutic ion beamMaeyama, Takuya*; Katsumura, Yosuke; Yamashita, Shinichi; Baldacchino, G.*; Taguchi, Mitsumasa; Kimura, Atsushi; Murakami, Takeshi*
no journal, ,
Solution of Coumarin-3-carboxylic acid (CCA) has been applied to yield measurement of 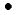 OH in water radiolysis with carbon ion beams. Production yield of a fluorescent probe, 7OH-CCA, which is a stable product produced after scavenging reaction for
OH in water radiolysis with carbon ion beams. Production yield of a fluorescent probe, 7OH-CCA, which is a stable product produced after scavenging reaction for 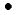 OH by CCA, was determined by using HPLC connected to a fluorometer. By using this chemical system,
OH by CCA, was determined by using HPLC connected to a fluorometer. By using this chemical system,  OH yields near the Bragg peak have been measured. Contribution of fragmentations, which are known to be significant near the Bragg peak of high-energy heavy ions, is also discussed by conducting fragmentation simulation with deterministic, semi-empirical and one-dimensional code named HIBRAC.
OH yields near the Bragg peak have been measured. Contribution of fragmentations, which are known to be significant near the Bragg peak of high-energy heavy ions, is also discussed by conducting fragmentation simulation with deterministic, semi-empirical and one-dimensional code named HIBRAC.
 OH yields estimated by PHITS
OH yields estimated by PHITSFuntowiez, D.*; Maeyama, Takuya*; Yamashita, Shinichi; Katsumura, Yosuke; Meesungnoen, J.*; Jay-Gerin, J.-P.*; Murakami, Takeshi*
no journal, ,
no abstracts in English
Yamashita, Shinichi; Katsumura, Yosuke; Lin, M.; Maeyama, Takuya*; Muroya, Yusa*; Murakami, Takeshi*
no journal, ,
no abstracts in English
Yamashita, Shinichi; Funtowiez, D.*; Maeyama, Takuya*; Midorikawa, Masamichi*; Oka, Toshitaka; Baldacchino, G.*; Taguchi, Mitsumasa; Kimura, Atsushi; Kudo, Hisaaki*; Katsumura, Yosuke; et al.
no journal, ,
Product yields of water radiolysis are inevitably important in understanding detailed mechanism of indirect action of ionizing radiations. We have conducted yield measurement of main products such as hydrated electron, hydroxyl radical ( OH) and hydrogen peroxide. We have also developed a sensitive method to determine
OH) and hydrogen peroxide. We have also developed a sensitive method to determine  OH yield with a fluorescent probe. In the method, aqueous solution of coumarin-3-carboxylic acid (CCA) is irradiated and
OH yield with a fluorescent probe. In the method, aqueous solution of coumarin-3-carboxylic acid (CCA) is irradiated and  OH is scavenged by CCA, resulting in production of fluorescent product 7OH-CCA. Applying this method,
OH is scavenged by CCA, resulting in production of fluorescent product 7OH-CCA. Applying this method,  OH yield near the Bragg peak has been measured in this study because the Bragg peak is overlapped cancer position in actual therapy. Beams of C 290 and 135 MeV/u and so on were taken for irradiation at the biological irradiation port at HIMAC facility of NIRS. It was found that
OH yield near the Bragg peak has been measured in this study because the Bragg peak is overlapped cancer position in actual therapy. Beams of C 290 and 135 MeV/u and so on were taken for irradiation at the biological irradiation port at HIMAC facility of NIRS. It was found that  OH yield show minimal value around the Bragg peak for every ion beam and it drastically jumps up several times at just downstream of the Bragg peak. It is already well known that contributions of fragmentation particles become non-negligible for high-energy heavy ions. Thus, further consideration including fragmentations is necessary to separate contributions of different fragmentation particles. Such consideration is being attempted by using simulations with HIBRAC and PHITS.
OH yield show minimal value around the Bragg peak for every ion beam and it drastically jumps up several times at just downstream of the Bragg peak. It is already well known that contributions of fragmentation particles become non-negligible for high-energy heavy ions. Thus, further consideration including fragmentations is necessary to separate contributions of different fragmentation particles. Such consideration is being attempted by using simulations with HIBRAC and PHITS.