Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 O
O submicron particles studied by polarized small-angle neutron scattering
submicron particles studied by polarized small-angle neutron scatteringNomura, Eiji*; Chiba, Momoko*; Matsuo, Sakoto*; Noda, Chiaki*; Kobayashi, Satoru*; Manjanna, J.*; Kawamura, Yukihiko*; Oishi, Kazuki*; Hiroi, Kosuke; Suzuki, Junichi*
AIP Advances (Internet), 12(3), p.035034_1 - 035034_5, 2022/03
Times Cited Count:3 Percentile:34.67(Nanoscience & Nanotechnology)Manjanna, J.*; Kozaki, Tamotsu*; Kozai, Naofumi; Sato, Seichi*
Journal of Nuclear Science and Technology, 44(7), p.929 - 932, 2007/07
Times Cited Count:18 Percentile:75.3(Nuclear Science & Technology)This study developed a new preparation method of Fe(II)-montmorillonite. In this method, first, a Fe(II)-NTA complex solution was prepared by dissolving iron oxides with NTA and a reducing agent. Next, montmorillonite was immersed in this Fe(II)-NTA solution to allow montmorillonite adsorb Fe ions. The advantages of this method are (1) inert-gas atmosphere is not needed for the entire preparation process and (2) no anions such as Cl
ions. The advantages of this method are (1) inert-gas atmosphere is not needed for the entire preparation process and (2) no anions such as Cl ions, which form ion pairs with Fe
ions, which form ion pairs with Fe ions, are not included in all the chemicals used.
ions, are not included in all the chemicals used.
Manjanna, J.*; Kozaki, Tamotsu*; Kozai, Naofumi; Sato, Seichi*
no journal, ,
In the repository of high level radioactive waste (HLW), the formation of Fe(II)-montmorillonite due to the interaction between overpack (carbon steel) and the surrounding buffer materials mainly composed of montmorillonite is an important issue to be investigated. However, preparation of Fe(II)-montmorillonite is difficult because of the difficulty in handling the redox sensitive Fe(II) species. In this study, a new method for preparation of Fe(II)-montmorillonite using Fe(II)-NTA(nitrilotriacetic acid) complex has been examined to overcome this problem. A Fe(II)-NTA complex solution was prepared by dissolving Fe O
O in an aqueous solution containing 20 mM NTA and 10 mM ascorbic acid. Adsorption of Fe
in an aqueous solution containing 20 mM NTA and 10 mM ascorbic acid. Adsorption of Fe ions on montmorillonite was carried out once by suspending montmorillonite in the Fe(II)-NTA solution with solid/liquid ratio of 1g/200 ml under Ar bubbling but without using an inert-gas glove-box. The prepared montmorillonite sample had a value of Fe
ions on montmorillonite was carried out once by suspending montmorillonite in the Fe(II)-NTA solution with solid/liquid ratio of 1g/200 ml under Ar bubbling but without using an inert-gas glove-box. The prepared montmorillonite sample had a value of Fe /Fe
/Fe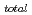 close to unity, showing that all the interlayer iron cations are divalent. The content of the exchanged iron was 97-100 meq/100g of the clay, indicating the dominant fraction of the cation exchange sites of the montmorillonite (about 116 meq/100g) are occupied with Fe
close to unity, showing that all the interlayer iron cations are divalent. The content of the exchanged iron was 97-100 meq/100g of the clay, indicating the dominant fraction of the cation exchange sites of the montmorillonite (about 116 meq/100g) are occupied with Fe ions. Thus we conclude that Fe(II)-montmorillonite could be successfully prepared by this method without an inert-gas glove-box. This could be one of the promising method to provide Fe(II)-montmorillonite for the studies related to safety assessment of the geological disposal of HLW.
ions. Thus we conclude that Fe(II)-montmorillonite could be successfully prepared by this method without an inert-gas glove-box. This could be one of the promising method to provide Fe(II)-montmorillonite for the studies related to safety assessment of the geological disposal of HLW.
Manjanna, J.*; Kozaki, Tamotsu*; Kozai, Naofumi; Sato, Seichi*
no journal, ,
Fe(II)-montmorillonite with Fe(II) ions occupying cation exchange sites is an ideal alteration product supposed to be formed by adsorption of corrosion products of overpack carbon steel in the buffer material bentonite after closing high-level radioactive waste disposal sites. Because Fe(II) ions are labile with the surrounding chemical conditions including pH and Eh, this study investigated the stability of Fe(II)-montmorillonite in purified water and Cr(VI) solutions. A Fe(II)-montmorillonite sample was prepared by the following two-step process. In the first-step, Fe(III)-montmorillonite was obtained by the conventional ion-exchange method with FeCl solution. Subsequently, it was reduced to Fe(II)-montmorillonite using ascorbic acid in aqueous medium. The stability of exchangeable Fe(II) in Fe(II)-montmorillonite was studied in distilled water (solid /liquid ratio: 0.5 g / 200 ml) with or without Ar bubbling. It took about 40 days to reach a Fe
solution. Subsequently, it was reduced to Fe(II)-montmorillonite using ascorbic acid in aqueous medium. The stability of exchangeable Fe(II) in Fe(II)-montmorillonite was studied in distilled water (solid /liquid ratio: 0.5 g / 200 ml) with or without Ar bubbling. It took about 40 days to reach a Fe /Fe
/Fe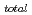 ratio of about 0.5 even without Ar bubbling, indicating that the exchangeable Fe(II) oxidizes very slowly. When Fe(II)-montmorillonite was suspended in 0.5 mM of K
ratio of about 0.5 even without Ar bubbling, indicating that the exchangeable Fe(II) oxidizes very slowly. When Fe(II)-montmorillonite was suspended in 0.5 mM of K Cr
Cr O
O solution (solid /liquid ratio: 0.1g clay/100 ml), more than 80 % of Cr(VI) was reduced within 10 min. No reduction of Cr(VI) was found for the cases of Fe(III)-montmorillonite samples. It can be considered that the exchangeable Fe(II) in Fe(II)-montmorillonite is relatively stable in water even without Ar bubbling but could reduce Cr(VI) with high reaction rate in an aqueous solution. This suggests the potential application of Fe(II)-montmorillonite for the environmental remediation of Cr(VI) contamination.
solution (solid /liquid ratio: 0.1g clay/100 ml), more than 80 % of Cr(VI) was reduced within 10 min. No reduction of Cr(VI) was found for the cases of Fe(III)-montmorillonite samples. It can be considered that the exchangeable Fe(II) in Fe(II)-montmorillonite is relatively stable in water even without Ar bubbling but could reduce Cr(VI) with high reaction rate in an aqueous solution. This suggests the potential application of Fe(II)-montmorillonite for the environmental remediation of Cr(VI) contamination.
Manjanna, J.*; Kozaki, Tamotsu*; Kozai, Naofumi; Sato, Seichi*
no journal, ,
Montmorillonite is the major clay mineral in bentonite, which is a promising candidate buffer material in geological disposal of high-level radioactive waste. After closing disposal sites, corrosion products of overpack carbon steel would alter characteristics of montmorillonite. One of the simply altered montmorillonite is Fe(II) or (III)-montmorillonite whose interlayer cations are Fe(II) or Fe(III). To clarify the nature of such altered montmorillonite, we prepared and characterized Fe(III)-montmorillonite. A Fe(III)-montmorillonite sample was prepared by an cation-exchange method with FeCl solutions. After the cation exchange, excess salt was removed by washing with de-ionized water and then the solid phase was vacuum dried. The total amount of iron extracted with (NH
solutions. After the cation exchange, excess salt was removed by washing with de-ionized water and then the solid phase was vacuum dried. The total amount of iron extracted with (NH )
) C
C O
O or Na
or Na EDTA solutions was 142 meq/100g-clay in terms of Fe(III) and slightly higher than the cation exchange capacity (CEC) of the montmorillonite (about 120 meq/100g-clay). This higher value than the CEC of the montmorillonite could be ascribed to the precipitation of amorphous iron (oxy) hydroxide on the cay surface, sorption of hydrolyzed species like Fe(OH)
EDTA solutions was 142 meq/100g-clay in terms of Fe(III) and slightly higher than the cation exchange capacity (CEC) of the montmorillonite (about 120 meq/100g-clay). This higher value than the CEC of the montmorillonite could be ascribed to the precipitation of amorphous iron (oxy) hydroxide on the cay surface, sorption of hydrolyzed species like Fe(OH) in the interlayer, or both. The results of thermal analysis, methylene blue adsorption, FT-IR analysis of this sample are described in this study.
in the interlayer, or both. The results of thermal analysis, methylene blue adsorption, FT-IR analysis of this sample are described in this study.