Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 MnO
MnO cathode in an all-solid-state thin-film battery during cycling
cathode in an all-solid-state thin-film battery during cyclingHikima, Kazuhiro*; Hinuma, Yoyo*; Shimizu, Keisuke*; Suzuki, Kota*; Taminato, So*; Hirayama, Masaaki*; Masuda, Takuya*; Tamura, Kazuhisa; Kanno, Ryoji*
ACS Applied Materials & Interfaces, 13(6), p.7650 - 7663, 2021/02
Times Cited Count:17 Percentile:65.38(Nanoscience & Nanotechnology)We evaluated the structural change of the cathode material Li MnO
MnO that was deposited as an epitaxial film with an (001) orientation in an all-solid-state battery. In case of the electrode with LiPO
that was deposited as an epitaxial film with an (001) orientation in an all-solid-state battery. In case of the electrode with LiPO coating. Experiments revealed a structural change to a high-capacity (activated) phase that proceeded gradually and continuously with cycling. The activated phase barely showed any capacity fading. We propose a mechanism of structural change with cycling: charging to a high voltage at a sufficiently low Li concentration typically induces irreversible transition to a phase detrimental to cycling that could, but not necessarily, be accompanied by the dissolution of Mn and/or the release of O into the electrolyte, while a gradual irreversible transition to an activated phase happens at a similar Li concentration under a lower voltage.
coating. Experiments revealed a structural change to a high-capacity (activated) phase that proceeded gradually and continuously with cycling. The activated phase barely showed any capacity fading. We propose a mechanism of structural change with cycling: charging to a high voltage at a sufficiently low Li concentration typically induces irreversible transition to a phase detrimental to cycling that could, but not necessarily, be accompanied by the dissolution of Mn and/or the release of O into the electrolyte, while a gradual irreversible transition to an activated phase happens at a similar Li concentration under a lower voltage.
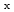 promoter on Pt electro-catalyst
promoter on Pt electro-catalystFugane, Keisuke*; Mori, Toshiyuki*; Yan, P.*; Masuda, Takuya*; Yamamoto, Shunya; Ye, F.*; Yoshikawa, Hideki*; Auchterlonie, G.*; Drennan, J.*
ACS Applied Materials & Interfaces, 7(4), p.2698 - 2707, 2015/02
Times Cited Count:31 Percentile:65.07(Nanoscience & Nanotechnology)no abstracts in English
Okawa, Yukihisa*; Masuda, Takuya*; Uehara, Hiromitsu*; Matsumura, Daiju; Tamura, Kazuhisa; Nishihata, Yasuo; Uosaki, Kohei*
RSC Advances (Internet), 3(35), p.15094 - 15101, 2013/09
Times Cited Count:9 Percentile:34.19(Chemistry, Multidisciplinary)Masuda, Takuya*; Fukumitsu, Hitoshi*; Kondo, Toshihiro*; Naohara, Hideo*; Tamura, Kazuhisa; Sakata, Osami*; Uosaki, Kohei*
Journal of Physical Chemistry C, 117(23), p.12168 - 12171, 2013/06
Times Cited Count:22 Percentile:54.95(Chemistry, Physical)The structure of the perfluorosulfonated ionomer (PFSI)/Pt(111) interface in a membrane electrode assembly (MEA)-like configuration of a polymer electrolyte membrane (PEM) fuel cell, that is, a vacuum evaporated Pt layer/PEM(Nafion membrane)/PFSI(adhesion Nafion layer)/Pt(111) single crystal, and its bias-induced change were investigated by surface X-ray scattering measurement at an atomic level. Crystal truncation rod measurement shows that PFSI adsorbed on the Pt(111)-(1 1) surface without bias. When the Pt(111) electrode was positively biased to form Pt oxide, the PFSI layer was detached from the Pt surface and oxygen atoms penetrated into the Pt lattice.
1) surface without bias. When the Pt(111) electrode was positively biased to form Pt oxide, the PFSI layer was detached from the Pt surface and oxygen atoms penetrated into the Pt lattice.
 nanocomposite electrocatalyst; An in situ electrochemical X-ray absorption fine structure study
nanocomposite electrocatalyst; An in situ electrochemical X-ray absorption fine structure studyMasuda, Takuya*; Fukumitsu, Hitoshi*; Fugane, Keisuke*; Togasaki, Hirotaka*; Matsumura, Daiju; Tamura, Kazuhisa; Nishihata, Yasuo; Yoshikawa, Hideki*; Kobayashi, Keisuke*; Mori, Toshiyuki*; et al.
Journal of Physical Chemistry C, 116(18), p.10098 - 10102, 2012/05
Times Cited Count:127 Percentile:93.68(Chemistry, Physical)In situ electrochemical X-ray absorption fine structure (XAFS) measurements were performed at the Pt L and Ce L
and Ce L edges of the Pt-CeO
edges of the Pt-CeO /C catalyst, which was prepared by a combined process of precipitation and coimpregnation methods, as well as at the Pt L
/C catalyst, which was prepared by a combined process of precipitation and coimpregnation methods, as well as at the Pt L edge of the conventional Pt/C catalyst in oxygen-saturated H
edge of the conventional Pt/C catalyst in oxygen-saturated H SO
SO solution to clarify the role of CeO
solution to clarify the role of CeO in the reduction of the overpotential for the oxygen reduction reaction (ORR) at the Pt-CeO
in the reduction of the overpotential for the oxygen reduction reaction (ORR) at the Pt-CeO nanocomposite compared with the conventional Pt/C catalyst. XAFS measurements clearly show that the enhancement of ORR activity is attributed to the inhibition of Pt oxide formation by the CeO
nanocomposite compared with the conventional Pt/C catalyst. XAFS measurements clearly show that the enhancement of ORR activity is attributed to the inhibition of Pt oxide formation by the CeO layer, of which Ce
layer, of which Ce was oxidized to Ce
was oxidized to Ce instead of Pt at the Pt oxide formation potential.
instead of Pt at the Pt oxide formation potential.
 based on the first-principles calculations
based on the first-principles calculationsIkaida, Riku*; Sano, Kazuhiro*; Masuda, Yoshimi*; Sekikawa, Takuya; Ono, Yoshiaki*
no journal, ,
The superconductivity of SrTiO , which is used in solar cells and as a photocatalyst, is observed in systems doped with electrons such as Nb, but it also exhibits crystal instability due to doping, and the realization of superconductivity is in competition with doping and crystal instability. Since the crystal instability is not well understood, we calculated the electronic structure of SrTiO
, which is used in solar cells and as a photocatalyst, is observed in systems doped with electrons such as Nb, but it also exhibits crystal instability due to doping, and the realization of superconductivity is in competition with doping and crystal instability. Since the crystal instability is not well understood, we calculated the electronic structure of SrTiO with electron doping as a parameter using the first-principles calculation software Quantum ESPRESSO. As a result, we succeeded in obtaining a solution for the decrease in frequency of crystal vibration (softening), which is considered to be caused by the ferroelectric transition, by reducing the amount of electron doping, and at the same time, we obtained the result that the superconducting transition temperature increases. This result is consistent with the experimental results of previous studies. This study provides new insight into the mechanism of superconductivity in SrTiO
with electron doping as a parameter using the first-principles calculation software Quantum ESPRESSO. As a result, we succeeded in obtaining a solution for the decrease in frequency of crystal vibration (softening), which is considered to be caused by the ferroelectric transition, by reducing the amount of electron doping, and at the same time, we obtained the result that the superconducting transition temperature increases. This result is consistent with the experimental results of previous studies. This study provides new insight into the mechanism of superconductivity in SrTiO , which has been an unsolved problem for many years.
, which has been an unsolved problem for many years.
 model interface for polymer electrolyte fuel cells
model interface for polymer electrolyte fuel cellsFugane, Keisuke*; Mori, Toshiyuki*; Masuda, Takuya*; Uosaki, Kohei*; Yamamoto, Shunya; Maekawa, Yasunari
no journal, ,
no abstracts in English
 for improvement of oxygen reduction reaction activity on Pt-CeO
for improvement of oxygen reduction reaction activity on Pt-CeO model electro catalysis
model electro catalysisFugane, Keisuke*; Mori, Toshiyuki*; Wu, Y. Y.*; Yamamoto, Shunya; Maekawa, Yasunari; Yoshikawa, Hideki*; Yamashita, Yoshiyuki*; Ueda, Shigenori*; Vladimir, M.*; Suzuki, Akira*; et al.
no journal, ,
no abstracts in English
 based on the first-principles calculations; Analysis using Tetrahedron Method II
based on the first-principles calculations; Analysis using Tetrahedron Method IIIkaida, Riku*; Sekikawa, Takuya; Ono, Yoshiaki*; Sano, Kazuhiro*; Masuda, Yoshimi*
no journal, ,
The SrTiO , a dilute mixture of Nb and other elements, has been studied as one of the most dilute free electron superconductors. At the same time, SrTiO
, a dilute mixture of Nb and other elements, has been studied as one of the most dilute free electron superconductors. At the same time, SrTiO has a huge dielectric constant at low temperatures, and ferroelectric transition occurs due to the substitution of O18 for O16 and Ca for Sr. Furthermore, an increase in the superconducting transition temperature Tc has been observed at substitution concentrations near where the ferroelectric transition occurs. However, the relationship between ferroelectricity and superconductivity is not clarified. In this study, we calculated the physical quantities related to ferroelectricity and superconductivity in the free electron density region near the highest superconducting transition temperature using the first-principles calculation software Quantum ESPRESSO. In the result, we found that the frequency of crystal oscillation decreases with decreasing free electron density, which is an indication of the ferroelectric transition. Furthermore, we found that Tc increases with decreasing free electron density. These results lead to a theoretical clarification of the relationship between ferroelectricity and superconductivity in SrTiO
has a huge dielectric constant at low temperatures, and ferroelectric transition occurs due to the substitution of O18 for O16 and Ca for Sr. Furthermore, an increase in the superconducting transition temperature Tc has been observed at substitution concentrations near where the ferroelectric transition occurs. However, the relationship between ferroelectricity and superconductivity is not clarified. In this study, we calculated the physical quantities related to ferroelectricity and superconductivity in the free electron density region near the highest superconducting transition temperature using the first-principles calculation software Quantum ESPRESSO. In the result, we found that the frequency of crystal oscillation decreases with decreasing free electron density, which is an indication of the ferroelectric transition. Furthermore, we found that Tc increases with decreasing free electron density. These results lead to a theoretical clarification of the relationship between ferroelectricity and superconductivity in SrTiO .
.