Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Kinoshita, Ryoma; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
Analytical Sciences, 39(9), p.1575 - 1583, 2023/09
Times Cited Count:0 Percentile:0.00(Chemistry, Analytical)Extraction of Rh from HCl can be performed by NTAamide(C6) (hexahexyl-nitrilotriacetamide) and other related compounds into n-dodecane. We use ion-pair extraction of anionic species of Rh-chloride and protonated extractant. Rh behave as anion in hydrochloric acid and the tertiary nitrogen atom in extractant may be protonated to produce the quaternary amine in acidic condition. From the present work, the maximum distribution ratio of Rh(III) is 16. The D(Rh) values are changeable during preparation of the aqueous solutions because different Rh-Cl-H O complexes are formed in HCl media and show the slow exchange rate between Cl and H
O complexes are formed in HCl media and show the slow exchange rate between Cl and H O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl
O. Using the UV spectrum, Rh-chloride solution having the peak of spectrum at 504 nm can be extracted effectively, where RhCl (H
(H O)
O) and RhCl
and RhCl (H
(H O)
O)
 exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
exist mainly from DFT calculation. Stoichiometry of one-one complex of Rh and NTAamide is obtained from slope analysis, and 85 mM of concentrated Rh ion can be extracted.
Sasaki, Yuji; Nakase, Masahiko*; Kaneko, Masashi; Kobayashi, Toru; Takeshita, Kenji*; Matsumiya, Masahiko*
Analytical Sciences, 5 Pages, 2023/00
Times Cited Count:0 Percentile:0.00(Chemistry, Analytical)We conducted three field researches on Ru-extraction, XANES, and DFT-calculation. The order of the distribution ratio, D(Ru), from acid, HCl  H
H SO
SO
 HNO
HNO
 HClO
HClO , by MIDOA is studied by XANES spectra, which indicates the valency change of Ru in HCl media and supports the ion pairing extraction of anionic Ru ion and cationic MIDOA. The same extractant trend, NTAamide
, by MIDOA is studied by XANES spectra, which indicates the valency change of Ru in HCl media and supports the ion pairing extraction of anionic Ru ion and cationic MIDOA. The same extractant trend, NTAamide  MIDOA
MIDOA  IDOA, due to D values as the energy gap of HOMO and LUMO could be found by DFT calculation, which suggests that the reaction heat has a positive correlation with extractability for extractant.
IDOA, due to D values as the energy gap of HOMO and LUMO could be found by DFT calculation, which suggests that the reaction heat has a positive correlation with extractability for extractant.
Nomizu, Daiki; Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Katsuta, Shoichi*
Journal of Radioanalytical and Nuclear Chemistry, 331(3), p.1483 - 1493, 2022/03
Times Cited Count:4 Percentile:74.52(Chemistry, Analytical)We studied the successive formation of water soluble DGA (diglycolamide) and DOODA (dioxaoctanediamide) for the mutual separation of Ln in this extraction system. TODGA (tetraoctyl-diglycolamide) and DOODA(C8) (tetraoctyl-dioxaoctanediamide) have the opposite trend to extract light and heavy Ln through Ln-patterns. Metal-complexes of two folding Ln ions with water-soluble DOODA and three folding with DGA are found and their observed formation constants are calculated. The suitable separation condition (aqueous phase: 30 mM DOODA(C2) in 1 M nitric acid, organic phase: 0.1 M TODGA in n-dodecane) of multi-stage extraction (10  10) is conducted. From the present work, it is clear that La, Pr and Nd are mainly present in aqueous phase, instead Sm-Dy exist in the organic phase.
10) is conducted. From the present work, it is clear that La, Pr and Nd are mainly present in aqueous phase, instead Sm-Dy exist in the organic phase.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Separation Science and Technology, 57(16), p.2543 - 2553, 2022/00
Times Cited Count:3 Percentile:38.23(Chemistry, Multidisciplinary)The mutual separation of actinides (An) from lanthanides (Ln) using the masking agent of DTPA (diethylenetriamine-pentaacetic acid) or DTBA (diethylenetriamine-triacetic acid-bis(diethylacetamide)) in the aqueous phase through DGA extraction, referring TALSPEAK method, is focused. We investigate to obtain the same separation performance using commercially available DTPA on that using DTBA. In this work, we select lactic acid (LA) of pH buffer from 10 organic acids and ethylenediamine (ED) for the pH adjustment. Almost the same D and SF values are obtained among the conditions: TODGA-DTPA-LA-NaOH, TODGA-DTPA-LA-ED, and TODGA-DTBA-LA. The experimental results using batchwise multi-stage extractions show the average yields of Ln (La to Gd) and Am to be 3.73 and 98.1% in the aqueous phase using DGA-DTPA-LA-ED, to be 3.1 and 97.0% using DGA-DTPA-LA-NaOH, and to be 1.61 and 98.7% using DGA-DTBA-LA.
Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Solvent Extraction and Ion Exchange, 40(6), p.620 - 640, 2022/00
Times Cited Count:1 Percentile:12.61(Chemistry, Multidisciplinary)Owing to the chemical behavior of trivalent lanthanide and actinide ions with similar ionic radii, realizing this separation is still challenging. All lanthanides, Am, and Cm can be extracted using diglycolamide (DGA), and relatively high An/Ln separation efficiencies have been obtained using diethylenetriamine-triacetic-bisamide (DTBA). To improve the previous results as well as the separation conditions, we used organic acids for pH adjustment. The advantages of this modification included low HNO , DTBA concentrations and pH stability owing to the addition of lactic acid. Under these modified conditions, the recovery rates observed were as follows: 97.1% for Nd with the co-existence of 1.59% Am in organic phase, and 98.4% for Am with the co-existence of 2.95% Nd in aqueous phase.
, DTBA concentrations and pH stability owing to the addition of lactic acid. Under these modified conditions, the recovery rates observed were as follows: 97.1% for Nd with the co-existence of 1.59% Am in organic phase, and 98.4% for Am with the co-existence of 2.95% Nd in aqueous phase.
 /Eu
/Eu selectivity with diethylenetriaminepentaacetic acid and its bisamide chelates.
selectivity with diethylenetriaminepentaacetic acid and its bisamide chelates.Kaneko, Masashi; Sasaki, Yuji; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Journal of Nuclear Science and Technology, 58(5), p.515 - 526, 2021/05
Times Cited Count:3 Percentile:34.17(Nuclear Science & Technology)Density-functional theory calculations were applied to molecular structure and complex formation reaction modelings of metal ion complexes with diethylenetriaminepentaacetic acid (DTPA) and its bisamide (DTPABA) chelates to understand the metal ions selectivity between Am and Eu
and Eu . The calculated complexes with DTPA and DTPABA chelates reproduced the coordination geometries of experimental crystal structures. Calculated Gibbs free energies of the complex formation reactions indicated that Am
. The calculated complexes with DTPA and DTPABA chelates reproduced the coordination geometries of experimental crystal structures. Calculated Gibbs free energies of the complex formation reactions indicated that Am ion forms higher stable complexes with both chelates than Eu
ion forms higher stable complexes with both chelates than Eu ion, being consistent with the experimental results. The higher Am
ion, being consistent with the experimental results. The higher Am selectivity over Eu
selectivity over Eu was suggested to originate in the larger bond overlap between Am
was suggested to originate in the larger bond overlap between Am 5f-orbital and N 2s, 2p-orbital. This mean that the covalent contribution between metal ion and donor atoms differentiates the complex formation stabilities, leading to the Am
5f-orbital and N 2s, 2p-orbital. This mean that the covalent contribution between metal ion and donor atoms differentiates the complex formation stabilities, leading to the Am /Eu
/Eu selectivity. We expect that this study contributes to systematize the origin of metal ions selectivity and to accelerate novel ligands exploration.
selectivity. We expect that this study contributes to systematize the origin of metal ions selectivity and to accelerate novel ligands exploration.
Sasaki, Yuji; Morita, Keisuke; Matsumiya, Masahiko*; Ono, Ryoma*; Shiroishi, Hidenobu*
JOM, 73(4), p.1037 - 1043, 2021/04
Times Cited Count:4 Percentile:41.70(Materials Science, Multidisciplinary)The separation of Dy from Nd is studied from the viewpoint of recycling Dy from Nd magnets. Both metals are lanthanide elements, which means their mutual separation is difficult because of their similar chemical behaviors. All lanthanide elements can be extracted easily by using tetradodecyl-diglycolamide (TDdDGA) extractants, and it has a relatively high separation factor (SF) between Dy and Nd (SF over 10). In the present study, by performing eight extraction steps with the organic phase (0.1M TDdDGA in dodecane), ten steps with an aqueous phase (0.7 M HNO with metals), and six steps with another aqueous phase (0.7 M HNO
with metals), and six steps with another aqueous phase (0.7 M HNO without metals), approximately 99% Dy was recovered into the organic phase with 1% co-extraction of Nd.
without metals), approximately 99% Dy was recovered into the organic phase with 1% co-extraction of Nd.
Matsumiya, Masahiko*; Nomizu, Daiki*; Tsuchida, Yusuke*; Sasaki, Yuji
Hydrometallurgy, 199, p.105539_1 - 105539_8, 2021/02
Times Cited Count:4 Percentile:31.70(Metallurgy & Metallurgical Engineering)The synergistic solvent extraction of lanthanide(III) with mixtures of di-(2-ethylhexyl)phosphoric acid (D2EHPA, A) and monoisodecyl phosphoric acid (MIDPA, B) in phosphonium-based ionic liquid was investigated. In the case of D2EHPA or MIDPA single extractant system, Ln(III) (Ln = Pr and Nd) was extracted as [LnA HA] or [LnB
HA] or [LnB HB], respectively, the extracted species of Tb(III) or Dy(III) were determined by slope analysis. According to the equilibrium constants (
HB], respectively, the extracted species of Tb(III) or Dy(III) were determined by slope analysis. According to the equilibrium constants (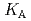 ,
, 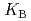 and
and 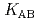 ) and the formation constants (
) and the formation constants (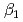 ,
, 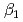 and
and 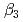 ), it was found that the extracted complex [TbHA
), it was found that the extracted complex [TbHA B
B or [DyHA
or [DyHA B
B ] was more stable than [LnA
] was more stable than [LnA HA] or [LnB
HA] or [LnB HB]. The synergistic extraction effects were investigated to study the possibility of separating Dy(III) from Pr(III) and Nd(III) according to their separation factors.
HB]. The synergistic extraction effects were investigated to study the possibility of separating Dy(III) from Pr(III) and Nd(III) according to their separation factors.
Matsumiya, Masahiko*; Tsuchida, Yusuke*; Sasaki, Yuji; Ono, Ryoma*; Nakase, Masahiko*; Takeshita, Kenji*
Journal of Radioanalytical and Nuclear Chemistry, 327(1), p.597 - 607, 2021/01
Times Cited Count:2 Percentile:23.17(Chemistry, Analytical)To achieve trichotomic separation of light lanthanides (Ln), heavy Ln, and Am, batchwise multi-stage extractions using tetraoctyl-diglycolamide (TODGA) extractant from organic acids are studied. Malonic acid (MA) has high solubility in water and is used as the main component of the aqueous phase. It is clear that the separation factor (SF) for Nd/Am from MA and that for La/Am from MA + HNO are both around 30. The light Ln (e.g., La and Ce) flowed-out in 1 M MA+0.05 M HNO
are both around 30. The light Ln (e.g., La and Ce) flowed-out in 1 M MA+0.05 M HNO (1st soln.), Am is recovered into 3 M MA (2nd soln.), and middle and heavy Ln (Nd and other heavy Ln) are back-extracted into 0.1 M TEDGA/water (3rd soln.). This extraction method can give 95% recovery of Am with total Ln of less than 16% present in high-level radioactive waste.
(1st soln.), Am is recovered into 3 M MA (2nd soln.), and middle and heavy Ln (Nd and other heavy Ln) are back-extracted into 0.1 M TEDGA/water (3rd soln.). This extraction method can give 95% recovery of Am with total Ln of less than 16% present in high-level radioactive waste.
 -diketone extractant and a neutral ligand
-diketone extractant and a neutral ligandMatsumiya, Masahiko*; Nomizu, Daiki*; Tsuchida, Yusuke*; Sasaki, Yuji
Solvent Extraction and Ion Exchange, 39(7), p.764 - 784, 2021/00
Times Cited Count:4 Percentile:28.73(Chemistry, Multidisciplinary)We investigated the solvent extraction of four rare earth (RE) elements (Pr, Nd, Tb, and Dy) from Nd-Fe-B magnets using mixtures of 1-(2-thienyl)-4,4,4,-trifluoro-1,3-butanedione (Htta) or 4,4,4-trifluoro-1-phenyl-1,3-butanedione (Hbfa) chelating extractants and tri-n-octylphosphine oxide (TOPO) neutral ligand in phosphonium based ionic liquids. A synergistic effect was observed for the extraction of the RE elements with the combination of extractant and neutral ligand. The separation of Tb(III) and Dy(III) from other RE(III) components was performed with seven extraction cycles.
Sasaki, Yuji; Matsumiya, Masahiko*; Tsuchida, Yusuke*
Analytical Sciences, 36(11), p.1303 - 1309, 2020/11
Times Cited Count:6 Percentile:33.32(Chemistry, Analytical)The mutual separation of lanthanides is studied by multi-stage extraction using extractant, DGA (diglycolamide) compounds. Tetraoctyl-DGA (TODGA) has a high extractability to lanthanides and relatively high separation factor (SF) between Dy and Nd (SF: over 20). The complete separation with such SF value can be achieved by multi-stage extraction. Less information on multi-stage extraction compared to batch extraction is presented up to now, thus we conduct the basic study about that. Confirming the experimental data to be identical to the calculation, the sample solution including both metals is employed for the batchwise multi-stage extraction. Ninety-seven % of Dy with under detection limit of Nd can be recovered into the organic phase from Nd with ten times higher concentration than Dy using the condition, 0.1 M TODGA/n-dodecane and 0.3 M HNO by multi-stage extraction of 9
by multi-stage extraction of 9 9 for organic and aqueous phases.
9 for organic and aqueous phases.
 -tetraoctyl-diglycolamide from organic acid
-tetraoctyl-diglycolamide from organic acidSasaki, Yuji; Matsumiya, Masahiko*; Nakase, Masahiko*; Takeshita, Kenji*
Chemistry Letters, 49(10), p.1216 - 1219, 2020/10
Times Cited Count:9 Percentile:43.66(Chemistry, Multidisciplinary)Lanthanide (Ln) extractions from organic acids to  -dodecane by
-dodecane by  -tetraoctyl-diglycolamide (TODGA) were conducted. Four organic acids (lactic acid, malonic acid, tartaric acid, and citric acid) were employed. Although these acids stabilize lanthanides in the aqueous phase, a distribution ratio (
-tetraoctyl-diglycolamide (TODGA) were conducted. Four organic acids (lactic acid, malonic acid, tartaric acid, and citric acid) were employed. Although these acids stabilize lanthanides in the aqueous phase, a distribution ratio ( ) greater 1 was obtained for heavy Ln. Ln patterns (
) greater 1 was obtained for heavy Ln. Ln patterns ( (Ln) against atomic number of Ln) show maximum values of Ho and Er. In order to obtain high
(Ln) against atomic number of Ln) show maximum values of Ho and Er. In order to obtain high  values, the addition of HNO
values, the addition of HNO in aqueous phase is found to be effective.
in aqueous phase is found to be effective.
 system
systemSasaki, Yuji; Morita, Keisuke; Matsumiya, Masahiko*; Nakase, Masahiko*
Radiochimica Acta, 108(9), p.689 - 699, 2020/09
Times Cited Count:9 Percentile:74.38(Chemistry, Inorganic & Nuclear)The simultaneous separation of Am and Cm from lanthanides is important for atomic energy fields. All lanthanides, Am, and Cm can be extracted by diglycolamide (DGA). In addition, relatively high separation factors between An and Ln were obtained by the extraction system of TODGA, DTPA (diethylenetriamine-pentaacetic acid) and HNO . In this work, DTPA-BA (diethylenetriamine-triacetic-bisamide), which is an improved version of DTPA, was employed for the separation of Ln and An. A relatively high separation factor (approximately 8) for actinides/lanthanides was obtained. Then, the multi-step extraction was performed. Thus, the recoveries of 94.7% for Nd and 4.7% for Am and Cm in organic phase, and 5.3% Nd and 95.3% for Am and Cm in aqueous phase were obtained.
. In this work, DTPA-BA (diethylenetriamine-triacetic-bisamide), which is an improved version of DTPA, was employed for the separation of Ln and An. A relatively high separation factor (approximately 8) for actinides/lanthanides was obtained. Then, the multi-step extraction was performed. Thus, the recoveries of 94.7% for Nd and 4.7% for Am and Cm in organic phase, and 5.3% Nd and 95.3% for Am and Cm in aqueous phase were obtained.
 -dioctylacetamide and direct electrodeposition from loaded organic phase
-dioctylacetamide and direct electrodeposition from loaded organic phaseMatsumiya, Masahiko*; Song, Y.*; Tsuchida, Yusuke*; Sasaki, Yuji
Separation and Purification Technology, 234, p.115841_1 - 115841_8, 2020/03
Times Cited Count:17 Percentile:60.62(Engineering, Chemical)The development of solvent extraction and direct electrodeposition processes is an important task to reduce the volume of secondary wastes. In this study, the extraction of Pd(II) from hydrochloric/chloride media using methylimino-bis- -dioctylacetamide (MIDOA) in three diluents (acetophenone; AP, 1,2-dichloroethane; DCE, or 1-octanol; OC) and the electrochemical behavior of the extracted Pd(II) complex in the MIDOA/AP bath was investigated. Pd(II) was found to be reduced to Pd(0) metal via a two-electron transfer between -2.38 V and -3.40 V. The potentiostatic electrodeposition of the extracted Pd(II) complex enabled us to recover the blackish electrodeposits, which were identified as Pd metal.
-dioctylacetamide (MIDOA) in three diluents (acetophenone; AP, 1,2-dichloroethane; DCE, or 1-octanol; OC) and the electrochemical behavior of the extracted Pd(II) complex in the MIDOA/AP bath was investigated. Pd(II) was found to be reduced to Pd(0) metal via a two-electron transfer between -2.38 V and -3.40 V. The potentiostatic electrodeposition of the extracted Pd(II) complex enabled us to recover the blackish electrodeposits, which were identified as Pd metal.
Sasaki, Yuji; Ban, Yasutoshi; Morita, Keisuke; Matsumiya, Masahiko*; Ono, Ryoma*; Shiroishi, Hidenobu*
Solvent Extraction Research and Development, Japan, 27(1), p.63 - 67, 2020/00
Times Cited Count:6 Percentile:30.44(Chemistry, Multidisciplinary)Mutual separation technique of Dy and Nd in Nd magnet is studied. Dy is more valuable than Nd, then Dy might be isolated and reused. Lanthanide elements can be extracted thoroughly by diglycolamide (DGA) extractants, we use this reagent for the recovery and isolation of Dy. Tetradodecyl-DGA (TDdDGA) has relatively high separation factors(SF) between Dy and Nd (SF=17-18) in HNO extraction system, counter-current extraction using TDdDGA was applied for their mutual separation. From the present study, using the condition, four extraction stages, organic phase: 0.1M TDdDGA in n-dodecane, aqueous phase: 0.3M HNO
extraction system, counter-current extraction using TDdDGA was applied for their mutual separation. From the present study, using the condition, four extraction stages, organic phase: 0.1M TDdDGA in n-dodecane, aqueous phase: 0.3M HNO , 92% Dy can be recovered with 0.7% co-extraction of Nd.
, 92% Dy can be recovered with 0.7% co-extraction of Nd.
Sasaki, Yuji; Morita, Keisuke; Matsumiya, Masahiko*; Nakase, Masahiko*
Proceedings of International Nuclear Fuel Cycle Conference / Light Water Reactor Fuel Performance Conference (Global/Top Fuel 2019) (USB Flash Drive), p.108 - 112, 2019/09
We attempted to separate An from Ln, and Am and Cm by the system including extractant and masking agent. The separation factor of Nd and Am was approximately 10 by TODGA-DTPA-BA and that of Am and Cm was over 3 by TODGA-DOODA(C2). Using these batch data, profiles of metal concentration with multi-step extractions proposed in this manuscript were demonstrated.
Sasaki, Yuji; Kaneko, Masashi; Ban, Yasutoshi; Kinoshita, Ryoma*; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
no journal, ,
We obtained relatively high D(Rh) of approximate 1 by iminodioctamide (IDOA) having tertially amino N atom from concentrated HCl solution. The reason is that IDOA is protonated, behaves as cationic extractant, and extracts anionic RhCl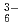 through ion-pair extraction. Up to now, there is less information on Rh(III) extraction, we investigate the behavior of Rh extraction and discuss with theoretical studies.
through ion-pair extraction. Up to now, there is less information on Rh(III) extraction, we investigate the behavior of Rh extraction and discuss with theoretical studies.
Kinoshita, Ryoma; Sasaki, Yuji; Kaneko, Masashi; Matsumiya, Masahiko*; Shinoku, Kota*; Shiroishi, Hidenobu*
no journal, ,
Many metal ions are stable in hydrochloric acid solutions as anionic species; diamidic-extractants containing tertiary amino nitrogen atom, such as NTAamide (hexaalkyl-nitrilotriacetamide), are normally protonated in acidic solutions and become cationic extractants. In this study, the ion-pair extraction reactions of metal chloride anions with cationic NTAamide(C6) extractant were investigated. In addition, we attempted to predict the distribution ratio by combining the stability constants of metal chloride complexes and DFT calculations, and compared the results with experimental values.
Sasaki, Yuji; Ban, Yasutoshi; Matsumiya, Masahiko*
no journal, ,
Metal ions are present as anionic species in hydrochloric acid. Diamino-extractants containing tertiary amino N atom, such as NTAamide, protonate in acidic solutions and become cationic extractants. In this study, the ion-pair extraction of metal chloride anions, especially platinum metals and the related metals, with the cationic extractants was performed and their extraction properties are studied.
Murakami, Sena; Sasaki, Yuji; Matsumiya, Masahiko*
no journal, ,
no abstracts in English