Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 -lactamase from the moderate halophile
-lactamase from the moderate halophile  sp.560 and the discovery of a Cs
sp.560 and the discovery of a Cs -selective binding site
-selective binding siteArai, Shigeki; Yonezawa, Yasushi*; Okazaki, Nobuo*; Matsumoto, Fumiko*; Shibazaki, Chie; Shimizu, Rumi; Yamada, Mitsugu*; Adachi, Motoyasu; Tamada, Taro; Kawamoto, Masahide*; et al.
Acta Crystallographica Section D, 71(3), p.541 - 554, 2015/03
Times Cited Count:8 Percentile:52.61(Biochemical Research Methods)The crystal structure of halophilic  -lactamase from
-lactamase from  sp.560 (HaBLA) was determined using X-ray crystallography. Moreover, the locations of bound Sr
sp.560 (HaBLA) was determined using X-ray crystallography. Moreover, the locations of bound Sr and Cs
and Cs ions were identified by anomalous X-ray diffraction. The location of one Cs
ions were identified by anomalous X-ray diffraction. The location of one Cs specific binding site was identified on HaBLA even in the presence of 9-fold molar excess of Na
specific binding site was identified on HaBLA even in the presence of 9-fold molar excess of Na (90 mM Na
(90 mM Na /10 mM Cs
/10 mM Cs ). This Cs
). This Cs binding site is formed by two main-chain O atoms and an aromatic ring of a side chain of Trp. An aromatic ring of Trp interacts with Cs
binding site is formed by two main-chain O atoms and an aromatic ring of a side chain of Trp. An aromatic ring of Trp interacts with Cs by the cation-
by the cation- interaction. The observation of a selective and high-affinity Cs
interaction. The observation of a selective and high-affinity Cs binding site provides important information that is useful for designing artificial Cs
binding site provides important information that is useful for designing artificial Cs binding sites useful in bioremediation of radioactive isotopes.
binding sites useful in bioremediation of radioactive isotopes.
 sp.593
sp.593Arai, Shigeki; Yonezawa, Yasushi*; Ishibashi, Matsujiro*; Matsumoto, Fumiko*; Adachi, Motoyasu; Tamada, Taro; Tokunaga, Hiroko*; Blaber, M.; Tokunaga, Masao*; Kuroki, Ryota
Acta Crystallographica Section D, 70(3), p.811 - 820, 2014/03
Times Cited Count:13 Percentile:63.53(Biochemical Research Methods)In order to clarify the structural basis of halophilic characteristics of an alkaline phosphatase derived from the moderate halophile  sp.593 (HaAP), the tertiary structure of HaAP was determined to 2.1
sp.593 (HaAP), the tertiary structure of HaAP was determined to 2.1 resolution by X-ray crystallography. Structural properties of surface negative charge and core hydrophobicity are shown to be intermediate between halophile and non-halophile characteristics, and may explain the unique functional adaptation to a wide-range of salt concentration.
resolution by X-ray crystallography. Structural properties of surface negative charge and core hydrophobicity are shown to be intermediate between halophile and non-halophile characteristics, and may explain the unique functional adaptation to a wide-range of salt concentration.

 reductase by X-ray crystallography; New insights into regulation of efficient electron transfer
reductase by X-ray crystallography; New insights into regulation of efficient electron transferYamada, Mitsugu*; Tamada, Taro; Takeda, Kazuki*; Matsumoto, Fumiko*; Ono, Hiraku*; Kosugi, Masayuki*; Takaba, Kiyofumi*; Shoyama, Yoshinari*; Kimura, Shigenobu*; Kuroki, Ryota; et al.
Journal of Molecular Biology, 425(22), p.4295 - 4306, 2013/11
Times Cited Count:24 Percentile:53.10(Biochemistry & Molecular Biology)NADH-Cytochrome 
 reductase (b5R), a flavoprotein consisting of NADH and flavin adenine dinucleotide (FAD) binding domains, catalyzes electron transfer from the two-electron carrier NADH to the one-electron carrier cytochrome
reductase (b5R), a flavoprotein consisting of NADH and flavin adenine dinucleotide (FAD) binding domains, catalyzes electron transfer from the two-electron carrier NADH to the one-electron carrier cytochrome 
 (Cb5). The crystal structures of both the fully reduced form and the oxidized form of porcine liver b5R were determined. In the reduced b5R structure determined at 1.68
(Cb5). The crystal structures of both the fully reduced form and the oxidized form of porcine liver b5R were determined. In the reduced b5R structure determined at 1.68 resolution, the relative configuration of the two domains was slightly shifted in comparison with that of the oxidized form. This shift resulted in an increase in the solvent-accessible surface area of FAD and created a new hydrogen-bonding interaction between the N5 atom of the isoalloxazine ring of FAD and the hydroxyl oxygen atom of Thr66, which is considered to be a key residue in the release of a proton from the N5 atom. The isoalloxazine ring of FAD in the reduced form is flat as in the oxidized form and stacked together with the nicotinamide ring of NAD
resolution, the relative configuration of the two domains was slightly shifted in comparison with that of the oxidized form. This shift resulted in an increase in the solvent-accessible surface area of FAD and created a new hydrogen-bonding interaction between the N5 atom of the isoalloxazine ring of FAD and the hydroxyl oxygen atom of Thr66, which is considered to be a key residue in the release of a proton from the N5 atom. The isoalloxazine ring of FAD in the reduced form is flat as in the oxidized form and stacked together with the nicotinamide ring of NAD . Determination of the oxidized b5R structure, including the hydrogen atoms, determined at 0.78
. Determination of the oxidized b5R structure, including the hydrogen atoms, determined at 0.78 resolution revealed the details of a hydrogen-bonding network from the N5 atom of FAD to His49 via Thr66. Both of the reduced and oxidized b5R structures explain how backflow in this catalytic cycle is prevented and the transfer of electrons to one-electron acceptors such as Cb5 is accelerated. Furthermore, crystallographic analysis by the cryo-trapping method suggests that re-oxidation follows a two-step mechanism. These results provide structural insights into the catalytic cycle of b5R.
resolution revealed the details of a hydrogen-bonding network from the N5 atom of FAD to His49 via Thr66. Both of the reduced and oxidized b5R structures explain how backflow in this catalytic cycle is prevented and the transfer of electrons to one-electron acceptors such as Cb5 is accelerated. Furthermore, crystallographic analysis by the cryo-trapping method suggests that re-oxidation follows a two-step mechanism. These results provide structural insights into the catalytic cycle of b5R.
 sp. nucleoside diphosphate kinase
sp. nucleoside diphosphate kinaseArai, Shigeki; Yonezawa, Yasushi; Okazaki, Nobuo; Matsumoto, Fumiko; Tamada, Taro; Tokunaga, Hiroko*; Ishibashi, Matsujiro*; Blaber, M.; Tokunaga, Masao*; Kuroki, Ryota
Protein Science, 21(4), p.498 - 510, 2012/04
Times Cited Count:15 Percentile:34.92(Biochemistry & Molecular Biology)In order to clarify the oligomer state of nucleoside diphosphate kinase (NDK) from moderately halophilic  sp. 593 (HaNDK), the crystal structure of HaNDK was determined by X-ray crystallography. The crystal structures of the wild-type HaNDK and the mutant HaNDK (E134A) showed a dimer and a tetramer, respectively. The higher ordered association of proteins usually contributes to an increase in thermal stability and substrate affinity. The change in the assembly form by a minimum mutation may be an effective way for NDK to acquire molecular characteristics suited to various circumstances.
sp. 593 (HaNDK), the crystal structure of HaNDK was determined by X-ray crystallography. The crystal structures of the wild-type HaNDK and the mutant HaNDK (E134A) showed a dimer and a tetramer, respectively. The higher ordered association of proteins usually contributes to an increase in thermal stability and substrate affinity. The change in the assembly form by a minimum mutation may be an effective way for NDK to acquire molecular characteristics suited to various circumstances.
Kimura, Tsunehisa*; Kimura, Fumiko*; Matsumoto, Kenji*; Metoki, Naoto
Neutron Diffraction, p.179 - 202, 2012/03
We report the results of the neutron scattering study on three dimensionally oriented pseudo single crystals under rotating magnetic field.
Fujiwara, Satoru; Plazanet, M.*; Matsumoto, Fumiko; Oda, Toshiro*
European Biophysics Journal, 40(5), p.661 - 671, 2011/05
Times Cited Count:11 Percentile:31.53(Biophysics)Matsumoto, Fumiko; Deshimaru, Shungo*; Oda, Toshiro*; Fujiwara, Satoru
Analytical Biochemistry, 399(2), p.299 - 301, 2010/04
Times Cited Count:2 Percentile:58.45(Biochemical Research Methods)We have developed a technique by which muscle thin filaments are reconstituted from the recombinant troponin components and the native thin filaments. By this technique, the reconstituted troponin complex is exchanged into the native thin filaments in the presence of 20% glycerol and 0.3 M KCl at pH 6.2. Over 90% of endogenous troponin complex was replaced with the recombinant troponin complex. Structural integrity and Ca -sensitivity of the reconstituted thin filament prepared by this technique was confirmed by X-ray fiber diffraction measurements and the thin filament activated myosin subfragment 1 ATPase measurements, respectively.
-sensitivity of the reconstituted thin filament prepared by this technique was confirmed by X-ray fiber diffraction measurements and the thin filament activated myosin subfragment 1 ATPase measurements, respectively.
Kimura, Fumiko*; Kimura, Tsunehisa*; Matsumoto, Kenji*; Metoki, Naoto
Crystal Growth & Design, 10(1), p.48 - 51, 2009/12
Times Cited Count:14 Percentile:75.71(Chemistry, Multidisciplinary)Single-crystal neutron diffraction analysis of an L-alanine pseudo single crystal (PSC, a composite in which microcrystals are aligned three-dimensionally) prepared from its powder is presented. Microcrystals of L-alanine were suspended in an ultraviolet-curable resin precursor and subjected to three-dimensional magnetic alignment, followed by consolidation of the resin precursor to fix the achieved alignment. A cylindrical PSC of diameter ca. 8 mm and height 10 mm was obtained and then subjected to neutron diffraction measurements. Pole figures measured at (040), (120), and (002) planes showed sharp peaks, demonstrating three-dimensional alignment of microcrystals. Scans were performed for 31 diffraction peaks whose intensities showed good agreement with calculation results. The present study showed the potential use of the PSC method in single-crystalneutron diffraction analysis when a large crystal is not available.
Matsumoto, Fumiko; Maeda, Kayo*; Chatake, Toshiyuki*; Maeda, Yuichiro*; Fujiwara, Satoru
Biochemical and Biophysical Research Communications, 382(1), p.205 - 209, 2009/04
Times Cited Count:15 Percentile:36.64(Biochemistry & Molecular Biology)Two cardiomyopathy-causing mutations, E244D and K247R, in human cardiac troponin T (TnT) are located in the coiled-coil region of the Tn-core domain. To elucidate effects of mutations in this region on the regulatory function of Tn, we measured Ca -dependent ATPase activity of myofibrils containing various mutants of TnT at these residues. The results confirmed that the mutant E244D increases the maximum ATPase activity without changing the Ca
-dependent ATPase activity of myofibrils containing various mutants of TnT at these residues. The results confirmed that the mutant E244D increases the maximum ATPase activity without changing the Ca sensitivity. The mutant K247R was shown for the first time to have the effect similar to the mutant E244D. Furthermore, various TnT mutants (E244D, E244M, E244A, E244K, K247R, K247E, and K247A) showed various effects on the maximum ATPase activity while the Ca
sensitivity. The mutant K247R was shown for the first time to have the effect similar to the mutant E244D. Furthermore, various TnT mutants (E244D, E244M, E244A, E244K, K247R, K247E, and K247A) showed various effects on the maximum ATPase activity while the Ca sensitivity was unchanged. Molecular dynamics simulations of the Tn-core containing these TnT mutants suggested that the hydrogen-bond network formed by the side chains of neighboring residues around residues 244 and 247 is important for Tn to function properly.
sensitivity was unchanged. Molecular dynamics simulations of the Tn-core containing these TnT mutants suggested that the hydrogen-bond network formed by the side chains of neighboring residues around residues 244 and 247 is important for Tn to function properly.
Fujiwara, Satoru; Plazanet, M.*; Matsumoto, Fumiko; Oda, Toshiro*
Biophysical Journal, 94(12), p.4880 - 4889, 2008/06
Times Cited Count:9 Percentile:20.78(Biophysics)Actin plays crucial roles in various aspects of cell motility. Flexibility of F-actin, a filamentous polymer formed by polymerization of the monomers (G-actin), is important for such a variety of functions. This flexibility allows F-actin to interact with various proteins, thereby expressing multiple functions. Understanding the variety of functions of actin thus requires understanding the flexibility of F-actin. As a first step towards this ultimate purpose, we carried out elastic incoherent neutron scattering (EINS) experiments on G-actin and F-actin under hydrated states. The mean square displacement (MSD) was estimated from the EINS measurements. Temperature dependence of MSD showed that two dynamical transitions occur at about 150 K and about 245 K, and that behavior of MSD is different between G-actin and F-actin, such that G-actin is "softer" than F-actin. The different behavior observed is ascribed to the differences in dynamical heterogeneity between F-actin and G-actin.
Fujiwara, Satoru; Matsumoto, Fumiko
Journal of Molecular Biology, 367(1), p.16 - 24, 2007/03
Times Cited Count:6 Percentile:9.93(Biochemistry & Molecular Biology)In striated muscles, contraction is regulated by the thin filament-based proteins, troponin consisting of three subunits (TnC, TnI, and TnT), and tropomyosin. Knowledge of in situ structures of these proteins is indispensable for elucidating this Ca -sensitive regulatory mechanism. We found from neutron scattering experiments that TnC within the thin filaments assumes extended dumbbell-like structures and moves toward the filament axis by binding of Ca
-sensitive regulatory mechanism. We found from neutron scattering experiments that TnC within the thin filaments assumes extended dumbbell-like structures and moves toward the filament axis by binding of Ca . Here, in order to obtain more detailed in situ structural information of TnC, neutron fiber diffraction measurements were performed. Neutron fiber diffraction patterns were obtained from the oriented samples of native thin filaments and the thin filaments containing deuterated TnC in the absence and presence of Ca
. Here, in order to obtain more detailed in situ structural information of TnC, neutron fiber diffraction measurements were performed. Neutron fiber diffraction patterns were obtained from the oriented samples of native thin filaments and the thin filaments containing deuterated TnC in the absence and presence of Ca . Analysis of the meridional reflections due to Tn-complex with aid of model calculation showed that the angle between the thin filament axis and the long axis of TnC was estimated to be 67(
. Analysis of the meridional reflections due to Tn-complex with aid of model calculation showed that the angle between the thin filament axis and the long axis of TnC was estimated to be 67( 7)
7) and 49(
and 49( 17)
17) , in the absence and presence of Ca
, in the absence and presence of Ca , respectively, suggesting that TnC, which assumes orientations rather perpendicular to the filament axis in the absence of Ca
, respectively, suggesting that TnC, which assumes orientations rather perpendicular to the filament axis in the absence of Ca , tilts toward the filament axis and the orientational and positional disorder increases by binding Ca
, tilts toward the filament axis and the orientational and positional disorder increases by binding Ca . It also showed that the relative position of the TnC moved by about 22
. It also showed that the relative position of the TnC moved by about 22 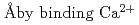 , and this apparent movement was concomitant with the movements of other Tn-subunits. This implies that by binding Ca
, and this apparent movement was concomitant with the movements of other Tn-subunits. This implies that by binding Ca , significant structural rearrangements of Tn-subunits occur.
, significant structural rearrangements of Tn-subunits occur.
Matsumoto, Fumiko*; Makino, Koji*; Maeda, Kayo*; Patzelt, H.*; Maeda, Yuichiro*; Fujiwara, Satoru
Journal of Molecular Biology, 342(4), p.1209 - 1221, 2004/09
Times Cited Count:19 Percentile:29.09(Biochemistry & Molecular Biology)Regulation of skeletal and cardiac muscle contraction is associated with the thin filament-based proteins, troponin C (TnC), TnI, TnT, tropomyosin, and actin. Knowledge of  structures of these proteins is indispensable for elucidating the molecular mechanism of this Ca
structures of these proteins is indispensable for elucidating the molecular mechanism of this Ca -sensitive regulation. Here the structure of TnC within the thin filaments was investigated with neutron scattering, combined with selective deuteration and the contrast matching technique. Deuterated TnC was prepared, reconstituted into the native thin filaments, and neutron scattering patterns of these reconstituted thin filaments containing deuterated TnC were measured under the condition where non-deuterated components were rendered 'invisible' to neutrons. The obtained scattering curves arising only from deuterated TnC were analyzed by model calculations using the Monte Carlo method. The results showed that upon binding of Ca
-sensitive regulation. Here the structure of TnC within the thin filaments was investigated with neutron scattering, combined with selective deuteration and the contrast matching technique. Deuterated TnC was prepared, reconstituted into the native thin filaments, and neutron scattering patterns of these reconstituted thin filaments containing deuterated TnC were measured under the condition where non-deuterated components were rendered 'invisible' to neutrons. The obtained scattering curves arising only from deuterated TnC were analyzed by model calculations using the Monte Carlo method. The results showed that upon binding of Ca ,
,  radius of gyration of TnC changed from 23 AA to 24 AA , and the radial position of TnC within the thin filament changed from 53 AA to 49 AA .
radius of gyration of TnC changed from 23 AA to 24 AA , and the radial position of TnC within the thin filament changed from 53 AA to 49 AA .
Fujiwara, Satoru; Matsumoto, Fumiko*; Yonezawa, Yasushige*
Journal of Molecular Biology, 331(1), p.21 - 28, 2003/08
Times Cited Count:46 Percentile:59.02(Biochemistry & Molecular Biology)The kinetic process of the fibril formation of hen egg white lysozyme (HEWL) in 90% ethanol in various salt concentrations has been investigated with time-resolved neutron scattering. It was shown that by addition of NaCl in a range between 0.3 mM and 1.0 mM, gelation occurred, and this gelation proceed through a two-step process; the lateral association of the protofilaments formed by HEWL, followed by the cross-linking of these fibrils formed. Both the structures of the fibrils and the rate of the gelation depended on NaCl concentration. Above 2 mM NaCl, precipitation occurred because of the formation of amorphous aggregates. Sensitivity of the aggregated structures to salt concentration suggests that electrostatic interaction plays an essential role in the formation of these structures. The structural diversity both in the fibrils and the aggregated structures of the fibrils can be interpreted in terms of the difference in the degree of the electrostatic shielding at different salt concentrations.
Fujiwara, Satoru; Plazanet, M.*; Matsumoto, Fumiko; Oda, Toshiro*
no journal, ,
no abstracts in English
Matsumoto, Fumiko; Maeda, Kayo*; Piroddi, N.*; Belus, A.*; Poggesi, C.*; Maeda, Yuichiro*; Fujiwara, Satoru
no journal, ,
Hypertrophic cardiomyopathy (HCM) is an autosomal dominant cardiac disease resulting from mutations in genes encoding contractile proteins, including troponin. HMC is characterized by functional aberration on the force-pCa relationship. Only a few HMC-causing mutations have been mapped on the coiled-coil region in the Tn core. Here we focus on two mutations in this region, E244D and K247R of TnT. Whereas E244D has been reported to show an increase of the maximum level of ATPase activity without changing the Ca sensitivity (Yanaga et. al., 1999), the functional consequence of K247R mutation has not been analyzed. We showed from the ATPase measurements of myofibrils containing the mutants K247R that this mutation exhibits similar functional consequences to the mutation E244D. Moreover, in order to understand how the mutations at these residues cause functional aberrations, we prepared various mutants of TnT (E244D, E244M, E244A, E244K, K247R, K247E, and K247A), having various volumes and charges, and measured ATPase activity of myofibrils containing these mutants TnT. The effect of mutations on the maximum ATPase activity level was different from each other while the Ca
sensitivity (Yanaga et. al., 1999), the functional consequence of K247R mutation has not been analyzed. We showed from the ATPase measurements of myofibrils containing the mutants K247R that this mutation exhibits similar functional consequences to the mutation E244D. Moreover, in order to understand how the mutations at these residues cause functional aberrations, we prepared various mutants of TnT (E244D, E244M, E244A, E244K, K247R, K247E, and K247A), having various volumes and charges, and measured ATPase activity of myofibrils containing these mutants TnT. The effect of mutations on the maximum ATPase activity level was different from each other while the Ca sensitivity was unchanged. To gain insight into the relationship between the ATPase activity and (possible) structural changes caused by these mutations, we carried out energy minimization/molecular dynamics calculations based on the crystal structure of the Tn-core. The results suggested that the stable hydrogen bond network at this region is important for the "proper" function of Tn.
sensitivity was unchanged. To gain insight into the relationship between the ATPase activity and (possible) structural changes caused by these mutations, we carried out energy minimization/molecular dynamics calculations based on the crystal structure of the Tn-core. The results suggested that the stable hydrogen bond network at this region is important for the "proper" function of Tn.
 2 chain (IL-13R
2 chain (IL-13R 2)
2)Matsumoto, Fumiko; Tamada, Taro; Honjo, Eijiro*; Ota, Shoichiro*; Izuhara, Kenji*; Kuroki, Ryota
no journal, ,
The allergic disease has dramatically increased in recent years. Interleukin-13 (IL-13) is a key cytokine critical to the development of T-cell-mediated humoral immune responses which are associated with allergy and asthma. IL-13 possesses two types of receptor: the heterodimer, composed of IL-13R 1 and IL-4R
1 and IL-4R , transducing the IL-13 signals; and the IL-13R
, transducing the IL-13 signals; and the IL-13R 2, acting as a nonsignaling "decoy" receptor. Although the extracellular region of IL-13R
2, acting as a nonsignaling "decoy" receptor. Although the extracellular region of IL-13R 1 and IL-13R
1 and IL-13R 2 are composed of a common structure of the class 1 cytokine receptor superfamily and they have similar amino acid sequence, the affinity of IL-13R
2 are composed of a common structure of the class 1 cytokine receptor superfamily and they have similar amino acid sequence, the affinity of IL-13R 2 with IL-13 is more than ten-fold higher than that of IL-13R
2 with IL-13 is more than ten-fold higher than that of IL-13R 1. In order to investigate the reason for this higher ligand affinity to IL-13R
1. In order to investigate the reason for this higher ligand affinity to IL-13R 2, extra cellular region of IL-13R
2, extra cellular region of IL-13R 2 was expressed by silkworm-baculovirus expression system after fusing the DNA cording the extracellular region of human IL-13R
2 was expressed by silkworm-baculovirus expression system after fusing the DNA cording the extracellular region of human IL-13R 2 with the Fc derived from mouse IgG2a. The expressed fusion protein IL-13R
2 with the Fc derived from mouse IgG2a. The expressed fusion protein IL-13R 2-Fc was purified by a protein G column, and the affinity to IL-13 was confirmed by gel filtration followed by light scattering analysis. After Fc region was removed by thrombin digestion, the resulting extra cellular region of IL-13R
2-Fc was purified by a protein G column, and the affinity to IL-13 was confirmed by gel filtration followed by light scattering analysis. After Fc region was removed by thrombin digestion, the resulting extra cellular region of IL-13R 2 receptor was confirmed to retain strong affinity to IL-13 with 1:1 ratio.
2 receptor was confirmed to retain strong affinity to IL-13 with 1:1 ratio.
Fujiwara, Satoru; Oda, Toshiro*; Plazanet, M.*; Matsumoto, Fumiko
no journal, ,
no abstracts in English
 sensitization in the myofibrils
sensitization in the myofibrilsMatsumoto, Fumiko; Maeda, Kayo*; Nitanai, Yasushi*; Oda, Toshiro*; Maeda, Yuichiro*; Fujiwara, Satoru
no journal, ,
Familial hypertrophic cardiomyopathy (HMC) has been reported to be caused by mutations in a regulatory protein, troponin (Tn). HMC is characterized by functional aberration on the force-pCa relationship. Only a few cardiomyopathy-causing mutations have been mapped on the coiled-coil region (IT-arm) in the Tn core domain. Here we focus on two mutations in the IT-arm, E244D and K247R of TnT. Whereas E244D has been reported to show an increase of the maximum level of ATPase activity, the functional consequence of K247R mutation has not been analyzed. In order to understand how these mutations cause functional aberrations, we measured ATPase activity of myofibrils containing various mutants of these residues (E244; D, M, A, K and K247; R, E, A). The maximum level of ATPase activity was found to increase in K247R, without changing the Ca ion sensitivity, as found in E244D. A close inspection of the crystal structure showed that the side chains of E244 and K247 form the triplet with that of E110 of TnI on the outside of the hydrophobic core of the coiled-coil. This triplet is thus likely to introduce flexibility into the IT-arm at this position. The mutations at the residues 244 and 247 could alter this flexibility. The results obtained here suggested that the proper flexibility of the IT-arm is important for the correct function of the myofibrils. The relationship between the IT-arm flexibility and functional aberration in the myofibril will be discussed.
Fujiwara, Satoru; Oda, Toshiro*; Plazanet, M.*; Matsumoto, Fumiko
no journal, ,
no abstracts in English
Fujiwara, Satoru; Matsumoto, Fumiko
no journal, ,
no abstracts in English