Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kobayashi, Taishi*; Sato, Yutaro*; Tonna, Ryutaro*; Matsumura, Daiju; Sasaki, Takayuki*; Ikeda, Atsushi
Dalton Transactions (Internet), 53(46), p.18616 - 18628, 2024/10
Times Cited Count:0 Percentile:0.00(Chemistry, Inorganic & Nuclear)Tsuji, Takuya; Matsumura, Daiju; Kobayashi, Toru
SPring-8/SACLA Riyo Kenkyu Seikashu (Internet), 12(5), p.259 - 262, 2024/10
no abstracts in English
Krajewska, A.*; Tsuji, Takuya; Matsumura, Daiju; 15 of others*
Science Advances (Internet), 10(41), p.eadn3880_1 - eadn3880_10, 2024/10
Times Cited Count:1 Percentile:0.00(Multidisciplinary Sciences) , AgNbO
, AgNbO , and KNbO
, and KNbO
Yoneda, Yasuhiro; Kobayashi, Toru; Tsuji, Takuya; Matsumura, Daiju; Saito, Yuji; Noguchi, Yuji*
Japanese Journal of Applied Physics, 63(9), p.09SP12_1 - 09SP12_10, 2024/09
Times Cited Count:1 Percentile:0.00(Physics, Applied)NaNbO , AgNbO
, AgNbO , and KNbO
, and KNbO with ABO
with ABO -type perovskite system is known to possess good ferroelectric properties. We directly determined the rattling space of each atom through local structure analysis. This analysis revealed the bonding sites with large fluctuations varied with varying ion size. Experimental evidence including soft X-ray absorption spectroscopy, indicates that the A-site ions are hybridized with oxygen.
-type perovskite system is known to possess good ferroelectric properties. We directly determined the rattling space of each atom through local structure analysis. This analysis revealed the bonding sites with large fluctuations varied with varying ion size. Experimental evidence including soft X-ray absorption spectroscopy, indicates that the A-site ions are hybridized with oxygen.

Yamamoto, Hajime*; Ikeda, Osamu*; Honda, Takashi*; Kimura, Kenta*; Aoyama, Takuya*; Ogushi, Kenya*; Suzuki, Akio*; Ishii, Kenji*; Matsumura, Daiju; Tsuji, Takuya; et al.
Physical Review Materials (Internet), 8(9), p.094402_1 - 094402_6, 2024/09
Times Cited Count:2 Percentile:56.91(Materials Science, Multidisciplinary)Jung, H.*; Matsumura, Daiju; 12 of others*
ACS Energy Letters (Internet), 9(5), p.2162 - 2172, 2024/04
Times Cited Count:10 Percentile:92.75(Chemistry, Physical)Lei, Y.-J.*; Matsumura, Daiju; 15 of others*
Nature Communications (Internet), 15, p.3325_1 - 3325_12, 2024/04
Times Cited Count:30 Percentile:98.78(Multidisciplinary Sciences)Nankawa, Takuya; Sekine, Yurina; Matsumura, Daiju; Hiroi, Kosuke; Takata, Shinichi; Kamiya, Yoshimi*; Honda, Takayuki*
Langmuir, 40(11), p.5725 - 5730, 2024/03
Times Cited Count:0 Percentile:0.00(Chemistry, Multidisciplinary)The chemical reaction between Fe and lacquer has been used to create the black color lacquer since ancient times. Here, the chemical state of Fe ions in black lacquer was investigated by using X-ray absorption near edge structure (XANES), extended X-ray absorption fine structure (EXAFS), and Fourier transform-infrared (FT-IR) spectroscopy. Fe(II) or Fe(III) was added to the lacquer paste to prepare black lacquer films by air drying, heating, or UV irradiation. The XANES spectral features of all the film samples were similar, meaning that the Fe ions in the samples existed in the trivalent state regardless of the oxidation state of the initially added Fe. The corresponding Fourier transforms of the EXAFS spectra around the Fe K-edge were used to investigate Fe sites in the lacquer films. The spectra of all the film samples were similar shapes, but the peak intensities decreased in the order air dried  heated
heated  UV irradiated films. This result indicates that heating and UV irradiation made the coordination structure of Fe in the lacquer non-uniform, and that heating caused the greatest non-uniformity. The complementary use of XANES, XAFS, and FT-IR spectroscopy is highly effective for non-destructive analysis of black lacquer in precious cultural artifacts.
UV irradiated films. This result indicates that heating and UV irradiation made the coordination structure of Fe in the lacquer non-uniform, and that heating caused the greatest non-uniformity. The complementary use of XANES, XAFS, and FT-IR spectroscopy is highly effective for non-destructive analysis of black lacquer in precious cultural artifacts.
Ikeda, Kazutaka*; Sashida, Sho*; Otomo, Toshiya*; Oshita, Hidetoshi*; Honda, Takashi*; Hawai, Takafumi*; Saito, Hiraku*; Ito, Shinichi*; Yokoo, Tetsuya*; Sakaki, Koji*; et al.
International Journal of Hydrogen Energy, 51(Part A), p.79 - 87, 2024/01
Times Cited Count:6 Percentile:48.53(Chemistry, Physical) Ga K-edge XANES study of Ga-exchanged zeolites at high temperatures under different atmospheres including vacuum, CO, and pressurized H
Ga K-edge XANES study of Ga-exchanged zeolites at high temperatures under different atmospheres including vacuum, CO, and pressurized H
Huang, M.*; Kinjo, Tetsuya*; Yasumura, Shunsaku*; Toyao, Takashi*; Matsumura, Daiju; Saito, Hiroyuki*; Shimizu, Kenichi*; Namiki, Norikazu*; Maeno, Zen*
Catalysis Science & Technology, 13(23), p.6832 - 6838, 2023/12
Times Cited Count:0 Percentile:0.00(Chemistry, Physical)Okazaki, Hiroyuki*; Idesaki, Akira*; Koshikawa, Hiroshi*; Matsumura, Daiju; Ikeda, Takashi*; Yamamoto, Shunya*; Yamaki, Tetsuya*
Journal of Physical Chemistry C, 127(49), p.23628 - 23633, 2023/12
Times Cited Count:1 Percentile:10.57(Chemistry, Physical) Na
Na TiO
TiO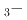 BaTiO
BaTiO solid solutions
solid solutionsYoneda, Yasuhiro; Kobayashi, Toru; Tsuji, Takuya; Matsumura, Daiju; Saito, Yuji; Noguchi, Yuji*
Japanese Journal of Applied Physics, 62(SM), p.SM1006_1 - SM1006_8, 2023/11
Times Cited Count:4 Percentile:50.15(Physics, Applied)Bi Na
Na TiO
TiO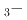 BaTiO
BaTiO (BNT
(BNT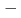 BT) solid solutions have been extensively studied because they exhibit good piezoelectric properties. In addition, a wide variety of phases are observed depending on the BT composition. Soft X-ray absorption spectroscopy and high-energy X-ray diffraction experiments of BNT
BT) solid solutions have been extensively studied because they exhibit good piezoelectric properties. In addition, a wide variety of phases are observed depending on the BT composition. Soft X-ray absorption spectroscopy and high-energy X-ray diffraction experiments of BNT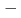 BT solid solutions were performed using synchrotron radiation. From the electronic structure and local structure of BNT
BT solid solutions were performed using synchrotron radiation. From the electronic structure and local structure of BNT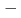 BT solid solution, the substitution effect of BT occurred mainly at the A site, which is the substitution site of Ba. The rhombohedral strain of the TiO
BT solid solution, the substitution effect of BT occurred mainly at the A site, which is the substitution site of Ba. The rhombohedral strain of the TiO octahedron did not change with the change in BT composition, suggesting that the change in the electronic structure at the O-
octahedron did not change with the change in BT composition, suggesting that the change in the electronic structure at the O- absorption edge is due to the change in the hybridization state.
absorption edge is due to the change in the hybridization state.
Matsumura, Daiju; Kimura, Yusaku*; Tsuji, Takuya; Mizuki, Junichiro*
SPring-8/SACLA Riyo Kenkyu Seikashu (Internet), 11(5), p.296 - 299, 2023/11
no abstracts in English
Ikeda, Yoichi*; Umemoto, Yoshihiko*; Matsumura, Daiju; Tsuji, Takuya; Hashimoto, Yuki*; Kitazawa, Takafumi*; Fujita, Masaki*
Materials Transactions, 64(9), p.2254 - 2260, 2023/09
Times Cited Count:4 Percentile:46.68(Materials Science, Multidisciplinary)Yoneda, Yasuhiro; Tsuji, Takuya; Matsumura, Daiju; Okamoto, Yoshihiro; Takaki, Seiya; Takano, Masahide
Physica B; Condensed Matter, 663, p.414960_1 - 414960_9, 2023/08
Times Cited Count:0 Percentile:0.00(Physics, Condensed Matter)We performed various synchrotron X-ray measurements to extract local and average structures of DyN-ZrN solid solutions. We performed the nanoscale structural analysis by combining X-ray absorption fine structure and high-energy X-ray diffraction. The DyN-ZrN solid solution has a rock-salt type cubic crystal structure, and there are instabilities such as the chemical order of the metal site and the distribution of the bond length of the nitrogen site.
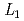 -edge XAFS measurement
-edge XAFS measurementTsuji, Takuya; Matsumura, Daiju; Kobayashi, Toru
SPring-8/SACLA Riyo Kenkyu Seikashu (Internet), 11(4), p.214 - 217, 2023/08
no abstracts in English
Inagawa, Kohei*; Matsumura, Daiju; Taniguchi, Masashi*; Uegaki, Shinya*; Nakayama, Tomohito*; Urano, Junnosuke*; Aotani, Takuro*; Tanaka, Hirohisa*
Journal of Physical Chemistry C, 127(24), p.11542 - 11549, 2023/06
Times Cited Count:2 Percentile:0.00(Chemistry, Physical) -edge X-ray absorption fine structure study of
-edge X-ray absorption fine structure study of 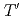 -type
-type 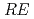
 CuO
CuO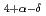 (
(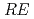 = Rare Earth); Toward unified understanding of electronic state of
= Rare Earth); Toward unified understanding of electronic state of 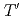 -type cuprate
-type cuprateChen, Y.*; Asano, Shun*; Wang, T.*; Xie, P.*; Kitayama, Shinnosuke*; Ishii, Kenji*; Matsumura, Daiju; Tsuji, Takuya; Taniguchi, Takanori*; Fujita, Masaki*
JPS Conference Proceedings (Internet), 38, p.011050_1 - 011050_6, 2023/05
Ouchi, Kazuki; Matsumura, Daiju; Tsuji, Takuya; Kobayashi, Toru; Otobe, Haruyoshi; Kitatsuji, Yoshihiro
RSC Advances (Internet), 13(24), p.16321 - 16326, 2023/05
Times Cited Count:1 Percentile:11.04(Chemistry, Multidisciplinary)We clarified the chemical state transformation of deposits following the reduction of uranyl ion (U O
O
 ) from the results of electrochemical quartz crystal microbalance, impedance spectra and X-ray absorption fine structure measurements. We propose the following deposition mechanism: (1) U
) from the results of electrochemical quartz crystal microbalance, impedance spectra and X-ray absorption fine structure measurements. We propose the following deposition mechanism: (1) U is formed by the disproportionation of U
is formed by the disproportionation of U . (2) U
. (2) U forms U
forms U hydroxide deposits, and (3) finally, the hydroxide deposits transform into U
hydroxide deposits, and (3) finally, the hydroxide deposits transform into U oxide, generally having a larger electrical resistance than the former.
oxide, generally having a larger electrical resistance than the former.
Ikeda, Yusuke*; Matsumura, Daiju; Tsuji, Takuya; Namai, Asuka*; Imoto, Kenta*; Tokoro, Hiroko*; Nakabayashi, Koji*; Okoshi, Shinichi*
Inorganica Chimica Acta, 550, p.121434_1 - 121434_8, 2023/03
Times Cited Count:0 Percentile:0.00(Chemistry, Inorganic & Nuclear)