Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Uchino, Seiko*; Narita, Hirokazu*; Kita, Keisuke*; Suzuki, Hideya*; Matsumura, Tatsuro; Naganawa, Hirochika*; Sakaguchi, Koichi*; Oto, Keisuke*
Solvent Extraction Research and Development, Japan, 30(1), p.39 - 46, 2023/00
The extraction of trivalent rare earth ions (RE ) from HNO
) from HNO solution using a triamide amine, tris(N,N-di-2-ethylhexyl-ethylamide)amine (DEHTAA), was conducted, and the extraction mechanism was estimated from extraction behavior of HNO
solution using a triamide amine, tris(N,N-di-2-ethylhexyl-ethylamide)amine (DEHTAA), was conducted, and the extraction mechanism was estimated from extraction behavior of HNO and RE
and RE and the relationship between atomic number and extraction percentages (E%) for RE
and the relationship between atomic number and extraction percentages (E%) for RE . A DEHTAA molecule dominantly formed a DEHTAA HNO
. A DEHTAA molecule dominantly formed a DEHTAA HNO at 1.0 M HNO
at 1.0 M HNO and a DEHTAA(HNO
and a DEHTAA(HNO )
) at 6.0 M HNO
at 6.0 M HNO in the acid-equilibrated organic phase. This would provide the unique dependence of E% for the light RE
in the acid-equilibrated organic phase. This would provide the unique dependence of E% for the light RE on the HNO
on the HNO concentration, in which the E% value had a minimum and maximum at
concentration, in which the E% value had a minimum and maximum at  0.5 M and
0.5 M and  2 M HNO
2 M HNO , respectively. The results of the slope analyses for the distribution ratios for RE
, respectively. The results of the slope analyses for the distribution ratios for RE suggested that the dominant RE
suggested that the dominant RE complex was RE(NO
complex was RE(NO )
) DEHTAA(DEHTAA HNO
DEHTAA(DEHTAA HNO ) at 1.0 M HNO
) at 1.0 M HNO . The E% for RE
. The E% for RE decreased from La
decreased from La to Lu
to Lu at 1.0 M HNO
at 1.0 M HNO ; on the other hand, those increased from La
; on the other hand, those increased from La to Nd
to Nd at 0.25 M and from La
at 0.25 M and from La to Sm
to Sm and 6.0 M HNO
and 6.0 M HNO .
.
Toigawa, Tomohiro; Kumagai, Yuta; Yamashita, Shinichi*; Ban, Yasutoshi; Matsumura, Tatsuro
UTNL-R-0502 (Internet), 2 Pages, 2022/04
This report summarizes the results obtained in FY2020 at the Electron Linac Facility of the University of Tokyo. The radiolysis process of  -hexaoctyl nitrilotriacetamide (HONTA), which is expected to be used as an extractant in a separation process for minor actinides, diluted in dodecane was investigated by pulse radiolysis experiments. The radical cation and the triplet-excited state of HONTA were observed in the nanosecond time region. The transition from the radical cation to the triplet excited state was slowed down by adding electron scavengers, and further, the reactivity of the triplet excited state was also suppressed.
-hexaoctyl nitrilotriacetamide (HONTA), which is expected to be used as an extractant in a separation process for minor actinides, diluted in dodecane was investigated by pulse radiolysis experiments. The radical cation and the triplet-excited state of HONTA were observed in the nanosecond time region. The transition from the radical cation to the triplet excited state was slowed down by adding electron scavengers, and further, the reactivity of the triplet excited state was also suppressed.
Sakamoto, Atsushi; Kibe, Satoshi*; Kawanobe, Kazunori*; Fujisaku, Kazuhiko*; Sano, Yuichi; Takeuchi, Masayuki; Suzuki, Hideya*; Tsubata, Yasuhiro; Ban, Yasutoshi; Matsumura, Tatsuro
JAEA-Research 2021-003, 30 Pages, 2021/06
Japan Atomic Energy Agency has been developing a solvent extraction process called SELECT to recover minor actinides (MA) from spent nuclear fuel. In the SELECT process, TDdDGA, HONTA, and ADAAM are used as the extractants for MA + Ln corecovery, MA/Ln separation and Am/Cm separation, respectively. These extractants do not contain phosphorus (P), and consist of carbon (C), hydrogen (H), oxygen (O), and nitrogen (N). In this study, in order to give beneficial information for designing flowsheet, the mass transfer coefficients of Ln between HNO solution and TDdDGA or HONTA / n-dodecane solvent were evaluated by the single drop technique. Prior to the evaluation of mass transfer coefficient, we had optimized the structure of the single drop apparatus to improve accuracy of the measurement. Based on the mass transfer coefficients obtained in HNO
solution and TDdDGA or HONTA / n-dodecane solvent were evaluated by the single drop technique. Prior to the evaluation of mass transfer coefficient, we had optimized the structure of the single drop apparatus to improve accuracy of the measurement. Based on the mass transfer coefficients obtained in HNO / TDdDGA-n-dodecane system, Ln behaviors in the counter-current extraction and back-extraction using mixer-settlers and centrifugal contactors were estimated by simple calculation, and they had a good agreement with our previous experimental results. We also confirmed the mass transfer coefficients of Ln in HNO
/ TDdDGA-n-dodecane system, Ln behaviors in the counter-current extraction and back-extraction using mixer-settlers and centrifugal contactors were estimated by simple calculation, and they had a good agreement with our previous experimental results. We also confirmed the mass transfer coefficients of Ln in HNO / HONTA - n-dodecane system are under 10
/ HONTA - n-dodecane system are under 10 m/s.
m/s.
 -octylnitrilo-triacetamide (HONTA) complexes of americium and europium
-octylnitrilo-triacetamide (HONTA) complexes of americium and europiumToigawa, Tomohiro; Peterman, D. R.*; Meeker, D. S.*; Grimes, T. S.*; Zalupski, P. R.*; Mezyk, S. P.*; Cook, A. R.*; Yamashita, Shinichi*; Kumagai, Yuta; Matsumura, Tatsuro; et al.
Physical Chemistry Chemical Physics, 23(2), p.1343 - 1351, 2021/01
Times Cited Count:14 Percentile:81.66(Chemistry, Physical)The candidate An(III)/Ln(III) separation ligand hexa- -octylnitrilo-triacetamide (HONTA) was irradiated under envisioned SELECT (Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation) process conditions using a solvent test loop in conjunction with cobalt-60 gamma irradiation. We demonstrate that HONTA undergoes exponential decay with increasing gamma dose to produce a range of degradation products which have been identified and quantified by HPLC-ESI-MS/MS techniques. The combination of HONTA destruction and degradation product ingrowth, particularly dioctylamine, negatively impacts the extraction and back-extraction of both americium and europium ions. The loss of HONTA was attributed to its reaction with the solvent (
-octylnitrilo-triacetamide (HONTA) was irradiated under envisioned SELECT (Solvent Extraction from Liquid waste using Extractants of CHON-type for Transmutation) process conditions using a solvent test loop in conjunction with cobalt-60 gamma irradiation. We demonstrate that HONTA undergoes exponential decay with increasing gamma dose to produce a range of degradation products which have been identified and quantified by HPLC-ESI-MS/MS techniques. The combination of HONTA destruction and degradation product ingrowth, particularly dioctylamine, negatively impacts the extraction and back-extraction of both americium and europium ions. The loss of HONTA was attributed to its reaction with the solvent ( -dodecane) radical cation of
-dodecane) radical cation of 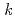 (HONTA + R
(HONTA + R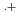 ) = (7.61
) = (7.61  0.82)
0.82)  10
10 M
M s
s obtained by pulse radiolysis techniques. However, when this ligand is bound to either americium or europium ions, the observed
obtained by pulse radiolysis techniques. However, when this ligand is bound to either americium or europium ions, the observed  -dodecane radical cation kinetics increase by over an order of magnitude. This large reactivity increase to additional reaction pathways occurring upon metal-ion binding. Lastly nanosecond time-resolved measurements showed that both direct and indirect HONTA radiolysis yielded the short-lived (
-dodecane radical cation kinetics increase by over an order of magnitude. This large reactivity increase to additional reaction pathways occurring upon metal-ion binding. Lastly nanosecond time-resolved measurements showed that both direct and indirect HONTA radiolysis yielded the short-lived ( 100 ns) HONTA radical cation as well as a longer-lived (
100 ns) HONTA radical cation as well as a longer-lived ( s) HONTA triplet excited state. These HONTA species are important precursors to the suite of HONTA degradation products observed.
s) HONTA triplet excited state. These HONTA species are important precursors to the suite of HONTA degradation products observed.
Toigawa, Tomohiro; Tsubata, Yasuhiro; Kai, Takeshi; Furuta, Takuya; Kumagai, Yuta; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 39(1), p.74 - 89, 2021/00
Times Cited Count:2 Percentile:9.60(Chemistry, Multidisciplinary)Absorbed-dose estimation is essential for evaluation of the radiation feasibility of minor-actinide-separation processes. We propose a dose-evaluation method based on radiation permeability, with comparisons of heterogeneous structures seen in the solvent-extraction process, such as emulsions forming in the mixture of the organic and aqueous phases. A demonstration of radiation-energy-transfer simulation is performed with a focus on the minor-actinide-recovery process from high-level liquid waste with the aid of the Monte Carlo radiation-transport code PHITS. The simulation results indicate that the dose absorbed by the extraction solvent from alpha ray depends upon the emulsion structure, and that from beta and gamma ray depends upon the mixer-settler-apparatus size. Non-negligible contributions of well-permeable gamma rays were indicated in terms of the plant operation of the minor-actinide-separation process.
Toigawa, Tomohiro; Murayama, Rin*; Kumagai, Yuta; Yamashita, Shinichi*; Suzuki, Hideya; Ban, Yasutoshi; Matsumura, Tatsuro
UTNL-R-0501, p.24 - 25, 2020/12
This report summarizes the results obtained in FY2019 at Electron Linac Facility of University of Tokyo. The radiolysis process of a diglycolamide extractant, which is expected to be used in the separation process of minor actinides (MA), in dodecane and octanol solutions was investigated by pulse radiolysis. As a result, it was suggested that by adding alcohol, the decomposition process of the diglycolamide extractant was different from the decomposition processes in the single solvent of dodecane considered that the decomposition occurred via a radical cation species of the extractant.
Kanamura, Shohei*; Takahashi, Yuya*; Omori, Takashi*; Nohira, Toshiyuki*; Sakamura, Yoshiharu*; Matsumura, Tatsuro
Denki Kagaku, 88(3), p.289 - 290, 2020/09
no abstracts in English
Nakamura, Satoshi; Kimura, Takahiro; Ban, Yasutoshi; Tsubata, Yasuhiro; Matsumura, Tatsuro
JAEA-Technology 2020-009, 22 Pages, 2020/08
Partitioning and transmutation technology division is planning to measure fission rate ratios that contribute to validate nuclear data of minor actinides (MA). For this purpose, MA sources for fission chambers were prepared using electrodeposition method. The radioactivity of each MA source was quantified, and its uncertainty was evaluated. Seven types of MA sources with different radioactivity were prepared using four nuclides of  Np,
Np,  Am,
Am,  Am, and
Am, and  Cm. A
Cm. A  Cm source solution of which radioactivity was quantified by isotope dilution method was used to prepare working standard sources of
Cm source solution of which radioactivity was quantified by isotope dilution method was used to prepare working standard sources of  Cm. The radioactivities were quantified as 1461 Bq, 2179 Bq, and 2938 Bq for
Cm. The radioactivities were quantified as 1461 Bq, 2179 Bq, and 2938 Bq for  Np sources, 1.428 MBq for
Np sources, 1.428 MBq for  Am source, 370.5 kBq and 89.57 kBq for
Am source, 370.5 kBq and 89.57 kBq for  Am sources, and 2.327 MBq for
Am sources, and 2.327 MBq for  Cm source with, the uncertainty of 0.35% (1
Cm source with, the uncertainty of 0.35% (1 ). This report summarizes the method for preparation and quantification of MA sources, and uncertainty evaluation.
). This report summarizes the method for preparation and quantification of MA sources, and uncertainty evaluation.
Tsutsui, Nao; Ban, Yasutoshi; Suzuki, Hideya*; Nakase, Masahiko*; Ito, Sayumi*; Inaba, Yusuke*; Matsumura, Tatsuro; Takeshita, Kenji*
Analytical Sciences, 36(2), p.241 - 246, 2020/02
Times Cited Count:22 Percentile:81.39(Chemistry, Analytical)To investigate the effective separation of actinides (Ans) from lanthanides (Lns), single-stage batch extraction experiments were performed with a novel extractant, tetradodecyl-1,10-phenanthroline-2,9-diamide (TDdPTDA) with various diluents such as 3-nitrobenzotrifluoride (F-3), nitrobenzene, and  -dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from
-dodecane for Am, Cm, and Lns. The extraction kinetics with TDdPTDA was rapid enough to perform the actual extraction flow sheet. The slopes of the distribution ratio versus TDdPTDA concentration and the distribution ratio versus nitric acid concentration were similar for F-3 and nitrobenzene systems but different from  -dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
-dodecane system. These differences were attributed to the characteristics of the diluents. This study reveals high distribution ratios of Am (
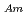 ) and Cm (
) and Cm (
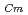 ) for TDdPTDA, with the high separation factors (
) for TDdPTDA, with the high separation factors ( s) of Am from Lns enough for their separation.
s) of Am from Lns enough for their separation.
Mishima, Ria; Inaba, Yusuke*; Tachioka, Sotaro*; Harigai, Miki*; Watanabe, Shinta*; Onoe, Jun*; Nakase, Masahiko*; Matsumura, Tatsuro; Takeshita, Kenji*
Chemistry Letters, 49(1), p.83 - 86, 2020/01
Times Cited Count:4 Percentile:20.20(Chemistry, Multidisciplinary)Separation of platinum group metals (PGMs) from high-level liquid waste generated from the reprocessing of spent nuclear fuels is important to produce good quality vitrified glass for final disposal. A new sorbent, Aluminum hexacyanoferrate (AlHCF), was synthesized and the general sorption behavior of PGMs from concentrated nitric acid was examined. Nitric acid caused substantial elution of AlHCF but the sorption of Pd stabilized the structure. Consequently, Rh was sorbed in the presence of Pd, whereas single Rh sorption caused complete dissolution of AlHCF. Relation between sorbed mount of Pd vs eluted Al and Fe revealed that the elution ratio of Al and Fe was not the same as molar ratio of synthesized AlHCF, indicating the re-sorption of Fe resulted in formation of new structure. The sorption mechanism of PGMs by this new sorbent, AlHCF, not only the simple ion exchange, but also oxidation reduction reaction as well as kinetics play important rule. Understanding the general sorption and dissolution behavior will help improve the sorption performance of PGMs by AlHCF.
Matsumura, Tatsuro
Kino Zairyo, 40(1), p.60 - 71, 2020/01
no abstracts in English
Tsukahara, Takehiko*; Saga, Kaname*; Suzuki, Hideya*; Matsumura, Tatsuro
Kurin Tekunoroji, 29(12), p.4 - 7, 2019/12
no abstracts in English
 -hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cell
-hexaoctyl nitrilotriacetamide (HONTA) using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya*; Hotoku, Shinobu; Tsutsui, Nao; Tsubata, Yasuhiro; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 37(7), p.489 - 499, 2019/11
Times Cited Count:17 Percentile:58.52(Chemistry, Multidisciplinary)A continuous counter-current experiment to separate minor actinides (MAs: Am and Cm) was performed with  -hexaochyl nitrilotriacetamide (HONTA) as an extractant. Nitric acid of 0.08 M (mol/dm
-hexaochyl nitrilotriacetamide (HONTA) as an extractant. Nitric acid of 0.08 M (mol/dm ) containing MAs and rare earths (REs) recovered from high-level waste was used as the Feed, and the experiment was conducted for 14 h. The ratios of Am and Cm recovered into the MA fraction measured 94.9% and 78.9%, respectively. HONTA hardly extracted Y, La, and Eu in the Feed (99.9% for Y, 99.9% for La, and 96.7% for Eu), most of which were distributed to the RE fraction. A portion of Nd was extracted by HONTA, and consequently the ratio of Nd in the RE fraction was 83.5%. The concentrations of MAs and some REs in each stage were calculated using a simulation code, and the results are consistent with the experimental values. This code indicates that the ratios of MAs in the MA fraction and REs in the RE fraction could be
) containing MAs and rare earths (REs) recovered from high-level waste was used as the Feed, and the experiment was conducted for 14 h. The ratios of Am and Cm recovered into the MA fraction measured 94.9% and 78.9%, respectively. HONTA hardly extracted Y, La, and Eu in the Feed (99.9% for Y, 99.9% for La, and 96.7% for Eu), most of which were distributed to the RE fraction. A portion of Nd was extracted by HONTA, and consequently the ratio of Nd in the RE fraction was 83.5%. The concentrations of MAs and some REs in each stage were calculated using a simulation code, and the results are consistent with the experimental values. This code indicates that the ratios of MAs in the MA fraction and REs in the RE fraction could be  99% by optimizing separation conditions.
99% by optimizing separation conditions.
Morita, Keisuke; Suzuki, Hideya; Matsumura, Tatsuro; Takahashi, Yuya*; Omori, Takashi*; Kaneko, Masaaki*; Asano, Kazuhito*
Proceedings of International Nuclear Fuel Cycle Conference / Light Water Reactor Fuel Performance Conference (Global/Top Fuel 2019) (USB Flash Drive), p.464 - 468, 2019/09
High level liquid waste (HLLW) contains several radionuclides with half-lives longer than 10 year. For reduce environmental burden of waste disposal, minor actinoids and long-lived fission products will to be partitioned and transmuted. JAEA and Toshiba developed process for recovering Se, Zr, Pd and Cs from HLLW. Solvent extraction for Zr with novel extractant,
year. For reduce environmental burden of waste disposal, minor actinoids and long-lived fission products will to be partitioned and transmuted. JAEA and Toshiba developed process for recovering Se, Zr, Pd and Cs from HLLW. Solvent extraction for Zr with novel extractant,  -didodecyl-2-hydroxyacetoamide (HAA) was detailed. The HAA system showed high selectivity for Zr, as indicated by the extraction order of Zr
-didodecyl-2-hydroxyacetoamide (HAA) was detailed. The HAA system showed high selectivity for Zr, as indicated by the extraction order of Zr  Mo
Mo  Pd
Pd  Ag
Ag  Sb
Sb  Sn
Sn  Lns
Lns  Fe. The extracted species was determined as Zr(HAA)
Fe. The extracted species was determined as Zr(HAA) (NO
(NO )
) (HNO
(HNO )
) . A continuous countercurrent extraction with HAA was applied to a simulated, concentrated HLLW after Pd, Se, and Cs removal, where the quantitative extraction of Zr and Mo was effectively demonstrated.
. A continuous countercurrent extraction with HAA was applied to a simulated, concentrated HLLW after Pd, Se, and Cs removal, where the quantitative extraction of Zr and Mo was effectively demonstrated.
 -trimethylglycine
-trimethylglycineSuzuki, Tomoya*; Narita, Hirokazu*; Ogata, Takeshi*; Suzuki, Hideya; Matsumura, Tatsuro; Kobayashi, Toru; Shiwaku, Hideaki; Yaita, Tsuyoshi
Solvent Extraction Research and Development, Japan, 26(1), p.11 - 19, 2019/06
Times Cited Count:3 Percentile:13.55(Chemistry, Multidisciplinary)The ability of AMP03, a styrene-divinylbenzene copolymer functionalized with  -trimethylglycine moieties, to adsorb Pd(II) from HNO
-trimethylglycine moieties, to adsorb Pd(II) from HNO solutions was investigated to elucidate the affinity of
solutions was investigated to elucidate the affinity of  -trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
-trimethylglycine for Pd(II). In the present study, we investigated the mechanism of Pd(II) adsorption by AMP03 by means of adsorption experiments, Fourier Transform Infrared (FT-IR) spectroscopy, and Extended X-ray Absorption Fine Structure (EXAFS) spectroscopy.
 -isopropylacrylamide) with diglycolamide-typed ligands
-isopropylacrylamide) with diglycolamide-typed ligandsSaga, Kaname*; Suzuki, Hideya; Matsumura, Tatsuro; Tsukahara, Takehiko*
Analytical Sciences, 35(4), p.461 - 464, 2019/04
Times Cited Count:3 Percentile:12.00(Chemistry, Analytical)The phase transition-based gelification phenomenon of poly- -isopropylacrylamide (PNIPAAm) in aqueous solutions has a great potential in developing new waste-free extraction processes of metal ions. By using hydrophobic diglycolamide-typed ligands in gelification extraction, a one-step complete extraction of all the RE ions from a nitric acid solution was successfully realized.
-isopropylacrylamide (PNIPAAm) in aqueous solutions has a great potential in developing new waste-free extraction processes of metal ions. By using hydrophobic diglycolamide-typed ligands in gelification extraction, a one-step complete extraction of all the RE ions from a nitric acid solution was successfully realized.
Tsukahara, Takehiko*; Suzuki, Hideya*; Matsumura, Tatsuro; Saga, Kaname*
Bunri Gijutsu, 49(4), p.221 - 225, 2019/04
no abstracts in English
 -tetradodecyldiglycolamide (TDdDGA) by using mixer-settler extractors in a hot cell
-tetradodecyldiglycolamide (TDdDGA) by using mixer-settler extractors in a hot cellBan, Yasutoshi; Suzuki, Hideya; Hotoku, Shinobu; Kawasaki, Tomohiro*; Sagawa, Hiroshi*; Tsutsui, Nao; Matsumura, Tatsuro
Solvent Extraction and Ion Exchange, 37(1), p.27 - 37, 2019/00
Times Cited Count:22 Percentile:67.14(Chemistry, Multidisciplinary)A continuous counter-current experiment using TDdDGA was performed using mixer-settler extractors installed in a hot cell. Nitric acid containing minor actinides (MAs: Am and Cm), rare earths (REs: Y, La, Nd, and Eu), and other fission products (Sr, Cs, Zr, Mo, Ru, Rh, and Pd) was fed to the extractor. TDdDGA effectively extracted MAs and REs from the feed, while other fission products were barely extracted. The extracted MAs and REs were back-extracted by bringing them in contact with 0.02 mol/dm nitric acid, and they were collected as the MA-RE fraction. The proportions of MA and RE in the MA-RE fraction were
nitric acid, and they were collected as the MA-RE fraction. The proportions of MA and RE in the MA-RE fraction were  98% and
98% and  86%, respectively. These results demonstrated the applicability of TDdDGA as an extractant for MAs and REs.
86%, respectively. These results demonstrated the applicability of TDdDGA as an extractant for MAs and REs.
Kaneko, Masashi; Suzuki, Hideya; Matsumura, Tatsuro
Inorganic Chemistry, 57(23), p.14513 - 14523, 2018/12
Times Cited Count:20 Percentile:77.49(Chemistry, Inorganic & Nuclear)We elucidated the separation mechanism between Am(III) and Cm(III) ions by using two different types of diamide ligands, diglycolamide (DGA) and alkylated diamide amine (ADAAM), by means of the density functional theory technique and electron density analysis. The molecular geometries and formation reactions of the metal-ligand complexes were modeled by using [M(DGA) ]
] and [M(ADAAM)(NO
and [M(ADAAM)(NO )
) (H
(H O)]. We successfully reproduced Cm(III) selectivity over Am(III) with DGA and Am(III) selectivity over Cm(III) with ADAAM. Furthermore, we analyzed the bonding properties between the metal ion and the diamide-type ligands by using model complexes, [M(DGA)
O)]. We successfully reproduced Cm(III) selectivity over Am(III) with DGA and Am(III) selectivity over Cm(III) with ADAAM. Furthermore, we analyzed the bonding properties between the metal ion and the diamide-type ligands by using model complexes, [M(DGA) ]
] and [M(ADAAM)(NO
and [M(ADAAM)(NO )
) (H
(H O)], and revealed the differences in terms of the bond dissociation energy and the metal 5f-orbital participation in the covalency between the Am(III) and the Cm(III) complexes. It was suggested that the differences were key factors to understand the Am(III)/Cm(III) selectivity.
O)], and revealed the differences in terms of the bond dissociation energy and the metal 5f-orbital participation in the covalency between the Am(III) and the Cm(III) complexes. It was suggested that the differences were key factors to understand the Am(III)/Cm(III) selectivity.
Fukaya, Yuji; Goto, Minoru; Ohashi, Hirofumi; Yan, X.; Nishihara, Tetsuo; Tsubata, Yasuhiro; Matsumura, Tatsuro
Journal of Nuclear Science and Technology, 55(11), p.1275 - 1290, 2018/11
Times Cited Count:0 Percentile:0.01(Nuclear Science & Technology)To reduce environmental burden and thread of nuclear proliferation, multi-recycling fuel cycle with High Temperature Gas-cooled Reactor (HTGR) has been investigated. Those problems are solved by incinerating TRans Uranium (TRU) nuclides, which is composed of plutonium and Minor Actinoide (MA), and there is concept to realize TRU incineration by multi-recycling with Fast Breeder Reactor (FBR). In this study, multi-recycling is realized even with thermal reactor by feeding fissile uranium from outside of the fuel cycle instead of breeding fissile nuclide. In this fuel cycle, recovered uranium by reprocessing and natural uranium are enriched and mixed with recovered TRU by reprocessing and partitioning to fabricate fresh fuels. The fuel cycle was designed for a Gas Turbine High Temperature Reactor (GTHTR300), whose thermal power is 600 MW, including conceptual design of uranium enrichment facility. Reprocessing is assumed as existing Plutonium Uranium Redox EXtraction (PUREX) with four-group partitioning technology. As a result, it was found that the TRU nuclides excluding neptunium can be recycled by the proposed cycle. The duration of potential toxicity decaying to natural uranium level can be reduced to approximately 300 years, and the footprint of repository for High Level Waste (HLW) can be reduced by 99.7% compared with GTHTR300 using existing reprocessing and disposal technology. Suppress plutonium is not generated from this cycle. Moreover, incineration of TRU from Light Water Reactor (LWR) cycle can be performed in this cycle.