Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nakagawa, Hiroshi; Matsuo, Tatsuhito*
Furontoranna Jikiden Sobunri Kaiseki Purotokoru; Jikken Igaku Bessatsu Saikyo No Suteppu UP Shirizu, p.222 - 227, 2022/07
Although the principle of neutron scattering is the same as that of X-ray scattering, it provides unique information due to the different interaction with the sample during the scattering process. Small-angle neutron scattering and quasi-elastic neutron scattering are promising methods to analyze the structure and association of proteins in droplets and their molecular dynamics. Neutron scattering experiments, which require nuclear reactors or accelerators as sources, are limited in Japan, and overseas facilities may be used if necessary.
Furuike, Yoshihiko*; Ouyang, D.*; Tominaga, Taiki*; Matsuo, Tatsuhito*; Mukaiyama, Atsushi*; Kawakita, Yukinobu; Fujiwara, Satoru*; Akiyama, Shuji*
Communications Physics (Internet), 5(1), p.75_1 - 75_12, 2022/04
Times Cited Count:7 Percentile:63.47(Physics, Multidisciplinary)Matsuo, Tatsuhito*; Nakagawa, Hiroshi
Hamon, 32(1), p.8 - 11, 2022/02
Incoherent neutron scattering is a powerful tool to investigate the dynamics of biomolecules and water. In this article, a new dynamical model called Matryoshka model is first described, which provides detailed information on the motions contained in the phospholipid molecules based on the scattering spectra. In the latter half of the article, a relationship between water activity and water mobility, and the effects of pressure and hydration on protein dynamics are discussed. These studies demonstrate the advancement of analytical methods which provide ever more detailed information for elucidating the mechanism of biological functions.
Nakagawa, Hiroshi; Matsuo, Tatsuhito*
Hamon, 32(1), p.12 - 15, 2022/02
To elucidate, predict, and control the biological functions of proteins, it is important to clarify their solution structure and dynamics. Small-angle scattering is one of effective methods for examining the changes in their structures and dynamics due to their binding with target molecules under physiological conditions, although its resolution is lower than that of crystallography, which provides structural information at atomic resolution. In addition, neutron spin echo is the only technique that can measure the conformational dynamics of proteins in the Q-region, which is covered by small-angle scattering. In this paper, we will introduce some of our recent studies using small-angle scattering and neutron spin echo.
 neutron scattering
neutron scatteringNakagawa, Hiroshi; Matsuo, Tatsuhito*
Jikken Igaku, 39(10), p.1667 - 1673, 2021/06
X-ray and neutron scattering are methods to reveal the structural state, assembly state, and intermolecular interactions of biomolecules in solution. Synchrotron X-rays provide highly accurate solution scattering data in a short time. Deuterated labeling can be used to make specific molecules invisible in neutron beams, allowing us to selectively observe only the molecules of interest in a molecular population. Neutron beams with energies comparable to thermal fluctuations are also suitable for measuring molecular motion. Although its application to the analysis of phase separation phenomena is currently limited, it is an effective method for analyzing phase separation states on the nano- to meso-scale and for studying the liquid-liquid phase separation.
Fujiwara, Satoru*; Matsuo, Tatsuhito*; Sugimoto, Yasunobu*; Shibata, Kaoru
Journal of Physical Chemistry Letters (Internet), 10(23), p.7505 - 7509, 2019/12
Times Cited Count:4 Percentile:17.43(Chemistry, Physical)Characterization of the dynamics of disordered polypeptide chains is required to elucidate the behavior of intrinsically disordered proteins and proteins under non-native states related to the folding process. Here we develop a method using quasielastic neutron scattering, combined with small-angle X-ray scattering and dynamic light scattering, to evaluate segmental motions of proteins as well as diffusion of the entire molecules and local side-chain motions. We apply this method to RNase A under the unfolded and molten-globule (MG) states. The diffusion coefficients arising from the segmental motions are evaluated and found to be different between the unfolded and MG states. The values obtained here are consistent with those obtained using the fluorescence-based techniques. These results demonstrate not only feasibility of this method but also usefulness to characterize the behavior of proteins under various disordered states.
 -synuclein related to propensity to amyloid fibril formation
-synuclein related to propensity to amyloid fibril formationFujiwara, Satoru*; Kono, Fumiaki*; Matsuo, Tatsuhito*; Sugimoto, Yasunobu*; Matsumoto, Tomoharu*; Narita, Tetsuhiro*; Shibata, Kaoru
Journal of Molecular Biology, 431(17), p.3229 - 3245, 2019/08
Times Cited Count:16 Percentile:49.25(Biochemistry & Molecular Biology) -synuclein (
-synuclein ( Syn) is an intrinsically disordered protein (IDP) with unknown function.
Syn) is an intrinsically disordered protein (IDP) with unknown function.  Syn is known to form amyloid fibrils, which are implicated with the pathogenesis of Parkinson's disease and other synucleinopathies. Elucidating the mechanism of fibril formation of
Syn is known to form amyloid fibrils, which are implicated with the pathogenesis of Parkinson's disease and other synucleinopathies. Elucidating the mechanism of fibril formation of  Syn is therefore important for understanding the mechanism of the pathogenesis of these diseases. Here, using the quasielastic neutron scattering (QENS) and small-angle X-ray scattering (SAXS) techniques, we investigated the dynamic and structural properties of
Syn is therefore important for understanding the mechanism of the pathogenesis of these diseases. Here, using the quasielastic neutron scattering (QENS) and small-angle X-ray scattering (SAXS) techniques, we investigated the dynamic and structural properties of  Syn. These results imply that fibril formation of
Syn. These results imply that fibril formation of  Syn requires not only the enhanced local motions but also the segmental motions such that the proper inter-molecular interactions are possible.
Syn requires not only the enhanced local motions but also the segmental motions such that the proper inter-molecular interactions are possible.
Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Nakajima, Kenji; Kawamura, Seiko; Kikuchi, Tatsuya; Fujiwara, Satoru
Biochemistry and Biophysics Reports (Internet), 6, p.220 - 225, 2016/07
 -synuclein studied by quasielastic neutron scattering
-synuclein studied by quasielastic neutron scatteringFujiwara, Satoru; Araki, Katsuya*; Matsuo, Tatsuhito; Yagi, Hisashi*; Yamada, Takeshi*; Shibata, Kaoru; Mochizuki, Hideki*
PLOS ONE (Internet), 11(4), p.e0151447_1 - e0151447_17, 2016/04
Times Cited Count:27 Percentile:63.33(Multidisciplinary Sciences)Matsuo, Tatsuhito; Takeda, Soichi*; Oda, Toshiro*; Fujiwara, Satoru
Biophysics and Physicobiology (Internet), 12, p.145 - 158, 2015/12
Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Nakajima, Kenji; Kawamura, Seiko; Kikuchi, Tatsuya; Fujiwara, Satoru
Biochemical and Biophysical Research Communications, 459(3), p.493 - 497, 2015/04
Times Cited Count:5 Percentile:15.05(Biochemistry & Molecular Biology)Matsuo, Tatsuhito; Arata, Toshiaki*; Oda, Toshiro*; Fujiwara, Satoru
Biophysics, 9, p.99 - 106, 2013/07
Matsuo, Tatsuhito; Natali, F.*; Plazanet, M.*; Zaccai, G.*; Fujiwara, Satoru
Journal of the Physical Society of Japan, 82(Suppl.A), p.SA020_1 - SA020_5, 2013/01
Times Cited Count:3 Percentile:26.25(Physics, Multidisciplinary)Troponin is a protein that regulates the muscle contraction depending on the intracellular Ca concentration. K247R mutation of TnT is known to cause the hypertrophic cardiomyopathy. In this work, neutron scattering was used to detect possible changes in dynamics of troponin caused by mutation. Elastic incoherent neutron scattering experiments were carried out on solution samples of the wild type, and K247R mutant at the IN13 spectrometer at the Institut Laue-Langevin, at temperatures between 280 K and 292 K with an interval of 3 K. From the measured scattering data, force constants (
concentration. K247R mutation of TnT is known to cause the hypertrophic cardiomyopathy. In this work, neutron scattering was used to detect possible changes in dynamics of troponin caused by mutation. Elastic incoherent neutron scattering experiments were carried out on solution samples of the wild type, and K247R mutant at the IN13 spectrometer at the Institut Laue-Langevin, at temperatures between 280 K and 292 K with an interval of 3 K. From the measured scattering data, force constants ( k
k ), which reflect the resilience of the protein, were calculated. The
), which reflect the resilience of the protein, were calculated. The  k
k values for the wild type and K247R mutant were 0.077 (0.035) N/m and 0.046 (0.026) N/m (mean(s.d.)), respectively. This suggests that the disease-causing mutant is more flexible than the wild type. The large flexibility might modulate Ca
values for the wild type and K247R mutant were 0.077 (0.035) N/m and 0.046 (0.026) N/m (mean(s.d.)), respectively. This suggests that the disease-causing mutant is more flexible than the wild type. The large flexibility might modulate Ca signal transmission mechanism, leading to the functional aberration.
signal transmission mechanism, leading to the functional aberration.
Yagi, Naoto*; Matsuo, Tatsuhito; Ota, Noboru*
Journal of Synchrotron Radiation, 19(4), p.574 - 578, 2012/07
Times Cited Count:1 Percentile:6.72(Instruments & Instrumentation)X-ray diffraction patterns were recorded from isolated single rod outer segments of frog. The outer segments in Ringer's solution were exposed to a 6  m microbeam (15 keV) at the BL40XU beamline, SPring-8. The diffraction pattern demonstrated a remarkable regularity in the stacking and flatness of the disk membranes. The electron density profile calculated from the intensity of up to tenth-order reflections showed a pair of bilayers that comprise a disk membrane. The structure of the disk membrane and the changes in the profile on swelling generally agreed with previous reports. Radiation damage was significant with an irradiation of 5
m microbeam (15 keV) at the BL40XU beamline, SPring-8. The diffraction pattern demonstrated a remarkable regularity in the stacking and flatness of the disk membranes. The electron density profile calculated from the intensity of up to tenth-order reflections showed a pair of bilayers that comprise a disk membrane. The structure of the disk membrane and the changes in the profile on swelling generally agreed with previous reports. Radiation damage was significant with an irradiation of 5  10
10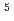 Gy which is much lower than the known damaging dose on proteins at the liquid-nitrogen temperature.
Gy which is much lower than the known damaging dose on proteins at the liquid-nitrogen temperature.
Nishiura, Masaya*; Toba, Shiori*; Takao, Daisuke*; Miyashiro, Daisuke*; Sakakibara, Hitoshi*; Matsuo, Tatsuhito; Kamimura, Shinji*; Oiwa, Kazuhiro*; Yagi, Naoto*; Iwamoto, Hiroyuki*
Journal of Structural Biology, 178(3), p.329 - 337, 2012/06
Times Cited Count:3 Percentile:8.24(Biochemistry & Molecular Biology)We report the first X-ray diffraction patterns recorded from single axonemes of eukaryotic flagella with a diameter of only  0.2
0.2  m, by using the technique of cryomicrodiffraction. A spermatozoon isolated from Drosophila melanogaster, was mounted straight in a glass capillary, quickly frozen and its 800-
m, by using the technique of cryomicrodiffraction. A spermatozoon isolated from Drosophila melanogaster, was mounted straight in a glass capillary, quickly frozen and its 800- m segment was irradiated end-on with intense synchrotron radiation X-ray microbeams (diameter, 2
m segment was irradiated end-on with intense synchrotron radiation X-ray microbeams (diameter, 2  m) at 74 K. Well-defined diffraction patterns were recorded, consisting of a large number of isolated reflection spots. The patterns had features of an 18-fold rotational symmetry as expected from the axonemal structure. The diffraction patterns were compared with the results of model calculations based on a published electron micrograph of the Drosophila axoneme. The comparison provided information on the native state of axoneme, including estimates of axonemal diameter and interdoublet spacing.
m) at 74 K. Well-defined diffraction patterns were recorded, consisting of a large number of isolated reflection spots. The patterns had features of an 18-fold rotational symmetry as expected from the axonemal structure. The diffraction patterns were compared with the results of model calculations based on a published electron micrograph of the Drosophila axoneme. The comparison provided information on the native state of axoneme, including estimates of axonemal diameter and interdoublet spacing.
Yamada, Yoshiteru*; Matsuo, Tatsuhito; Iwamoto, Hiroyuki*; Yagi, Naoto*
Biochemistry, 51(19), p.3963 - 3970, 2012/05
Times Cited Count:23 Percentile:49.57(Biochemistry & Molecular Biology)Calmodulin undergoes characteristic conformational changes by binding Ca . We measured the conformational changes of calmodulin upon Ca
. We measured the conformational changes of calmodulin upon Ca and mastoparan binding using the time-resolved small-angle X-ray scattering technique combined with flash photolysis of caged calcium. This measurement system covers the time range of 0.5-180 ms. Within 10 ms of the stepwise increase in Ca
and mastoparan binding using the time-resolved small-angle X-ray scattering technique combined with flash photolysis of caged calcium. This measurement system covers the time range of 0.5-180 ms. Within 10 ms of the stepwise increase in Ca concentration, we identified a distinct compact conformational state with a drastically different molecular dimension. This process is too fast to study with a conventional stopped-flow apparatus. The compact conformational state was also observed without mastoparan, indicating that the calmodulin forms a compact globular conformation by itself upon Ca
concentration, we identified a distinct compact conformational state with a drastically different molecular dimension. This process is too fast to study with a conventional stopped-flow apparatus. The compact conformational state was also observed without mastoparan, indicating that the calmodulin forms a compact globular conformation by itself upon Ca binding. This new conformational state of calmodulin seems to regulate Ca
binding. This new conformational state of calmodulin seems to regulate Ca binding and conformational changes in the N-terminal domain.
binding and conformational changes in the N-terminal domain.
Fujiwara, Satoru; Yamada, Takeshi*; Matsuo, Tatsuhito; Takahashi, Nobuaki; Kamazawa, Kazuya*; Kawakita, Yukinobu; Shibata, Kaoru*
no journal, ,
no abstracts in English
Fujiwara, Satoru; Yamada, Takeshi*; Matsuo, Tatsuhito; Takahashi, Nobuaki; Kamazawa, Kazuya*; Kawakita, Yukinobu; Shibata, Kaoru*
no journal, ,
 -synuclein; Implication for propensity for amyloid fibril formation
-synuclein; Implication for propensity for amyloid fibril formationFujiwara, Satoru; Araki, Katsuya*; Matsuo, Tatsuhito; Yagi, Hisashi*; Yamada, Takeshi*; Shibata, Kaoru; Mochizuki, Hideki*
no journal, ,
Fujiwara, Satoru; Yamada, Takeshi*; Matsuo, Tatsuhito; Shibata, Kaoru
no journal, ,