Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ohshima, Hiroyuki; Morishita, Masaki*; Aizawa, Kosuke; Ando, Masanori; Ashida, Takashi; Chikazawa, Yoshitaka; Doda, Norihiro; Enuma, Yasuhiro; Ezure, Toshiki; Fukano, Yoshitaka; et al.
Sodium-cooled Fast Reactors; JSME Series in Thermal and Nuclear Power Generation, Vol.3, 631 Pages, 2022/07
This book is a collection of the past experience of design, construction, and operation of two reactors, the latest knowledge and technology for SFR designs, and the future prospects of SFR development in Japan. It is intended to provide the perspective and the relevant knowledge to enable readers to become more familiar with SFR technology.
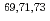 Co
CoLokotko, T.*; Leblond, S.*; Lee, J.*; Doornenbal, P.*; Obertelli, A.*; Poves, A.*; Nowacki, F.*; Ogata, Kazuyuki*; Yoshida, Kazuki; Authelet, G.*; et al.
Physical Review C, 101(3), p.034314_1 - 034314_7, 2020/03
Times Cited Count:12 Percentile:74.01(Physics, Nuclear)The structures of the neutron-rich 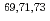 Co isotopes were investigated via (
Co isotopes were investigated via (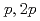 ) knockout reactions at the Radioactive Isotope Beam Factory, RIKEN. Level schemes were reconstructed using the
) knockout reactions at the Radioactive Isotope Beam Factory, RIKEN. Level schemes were reconstructed using the  coincidence technique, with tentative spin-parity assignments based on the measured inclusive and exclusive cross sections. Comparison with shell-model calculations suggests coexistence of spherical and deformed shapes at low excitation energies in the
coincidence technique, with tentative spin-parity assignments based on the measured inclusive and exclusive cross sections. Comparison with shell-model calculations suggests coexistence of spherical and deformed shapes at low excitation energies in the 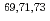 Co isotopes.
Co isotopes.
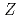 =28 and
=28 and  =50; Spectroscopy of
=50; Spectroscopy of 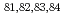 Zn
ZnShand, C. M.*; Podoly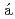 k, Zs.*; G
k, Zs.*; G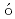 rska, M.*; Doornenbal, P.*; Obertelli, A.*; Nowacki, F.*; Otsuka, T.*; Sieja, K.*; Tostevin, J. A.*; Tsunoda, T.*; et al.
rska, M.*; Doornenbal, P.*; Obertelli, A.*; Nowacki, F.*; Otsuka, T.*; Sieja, K.*; Tostevin, J. A.*; Tsunoda, T.*; et al.
Physics Letters B, 773, p.492 - 497, 2017/10
Times Cited Count:28 Percentile:87.72(Astronomy & Astrophysics) Si
Si and YbT
and YbT Zn
Zn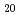 (T: Co, Rh, Ir)
(T: Co, Rh, Ir)Onuki, Yoshichika; Yasui, Shinichi*; Matsushita, Masaki*; Yoshiuchi, Shingo*; Oya, Masahiro*; Hirose, Yusuke*; Dung, N. D.*; Honda, Fuminori*; Takeuchi, Tetsuya*; Settai, Rikio*; et al.
Journal of the Physical Society of Japan, 80(Suppl.A), p.SA003_1 - SA003_6, 2011/12
 Fe
Fe O
O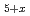 F
F (x
(x 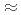 0.44)
0.44)Tsujimoto, Yoshihiro*; Yamaura, Kazunari*; Hayashi, Naoaki*; Kodama, Katsuaki; Igawa, Naoki; Matsushita, Yoshitaka*; Katsuya, Yoshio*; Shirako, Yuichi*; Akaogi, Masaki*; Muromachi, Eiji*
Chemistry of Materials, 23(16), p.3652 - 3658, 2011/08
Times Cited Count:29 Percentile:62.70(Chemistry, Physical)Topotactic reaction of the Ruddlesden-Popper phase Sr Fe
Fe O
O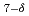 (
(
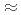 0.18) with polytetrafluoroethylene yields a highly fluorinated phase Sr
0.18) with polytetrafluoroethylene yields a highly fluorinated phase Sr Fe
Fe O
O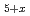 F
F (x
(x 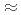 0.44), compared with Sr
0.44), compared with Sr Fe
Fe O
O F
F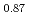 prepared by the reaction of Sr
prepared by the reaction of Sr Fe
Fe O
O and F
and F gas. Structure analyses based on powder neutron diffraction, synchrotron powder diffraction, and
gas. Structure analyses based on powder neutron diffraction, synchrotron powder diffraction, and 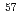 Fe M
Fe M ssbauer spectroscopy measurements demonstrate that the new oxyfluoride perovskite has no anion deficiencies and adopts the tetragonal structure (space group
ssbauer spectroscopy measurements demonstrate that the new oxyfluoride perovskite has no anion deficiencies and adopts the tetragonal structure (space group 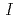 4/
4/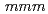 ) with lattice constants
) with lattice constants  = 3.87264(6)
= 3.87264(6)  and
and  = 21.3465(6)
= 21.3465(6)  at room temperature. The fluoride ions preferentially occupy the terminal apical anion sites with oxide ions in a disordered manner, which results in square pyramidal coordination around iron.
at room temperature. The fluoride ions preferentially occupy the terminal apical anion sites with oxide ions in a disordered manner, which results in square pyramidal coordination around iron.
 Zn
Zn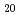
Onuki, Yoshichika; Yasui, Shinichi*; Yoshiuchi, Shingo*; Oya, Masahiro*; Matsushita, Masaki*; Hirose, Yusuke*; Takeuchi, Tetsuya*; Honda, Fuminori*; Settai, Rikio*; Sugiyama, Kiyohiro*; et al.
Journal of Physics; Conference Series, 273, p.012013_1 - 012013_4, 2011/02
Times Cited Count:1 Percentile:32.53(Physics, Condensed Matter) Zn
Zn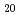
Takeuchi, Tetsuya*; Yasui, Shinichi*; Toda, Masatoshi*; Matsushita, Masaki*; Yoshiuchi, Shingo*; Oya, Masahiro*; Katayama, Keisuke*; Hirose, Yusuke*; Yoshitani, Naohisa*; Honda, Fuminori*; et al.
Journal of the Physical Society of Japan, 79(6), p.064609_1 - 064609_15, 2010/06
Times Cited Count:43 Percentile:83.25(Physics, Multidisciplinary) , CeCd
, CeCd , and PrCd
, and PrCd with a caged crystal structure
with a caged crystal structureYoshiuchi, Shingo*; Takeuchi, Tetsuya*; Oya, Masahiro*; Katayama, Keisuke*; Matsushita, Masaki*; Yoshitani, Naohisa*; Nishimura, Naoto*; Ota, Hisashi*; Tateiwa, Naoyuki; Yamamoto, Etsuji; et al.
Journal of the Physical Society of Japan, 79(4), p.044601_1 - 044601_11, 2010/04
Times Cited Count:15 Percentile:63.91(Physics, Multidisciplinary) Zn
Zn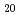
Yoshiuchi, Shingo*; Toda, Masatoshi*; Matsushita, Masaki*; Yasui, Shinichi*; Hirose, Yusuke*; Oya, Masahiro*; Katayama, Keisuke*; Honda, Fuminori*; Sugiyama, Kiyohiro*; Hagiwara, Masayuki*; et al.
Journal of the Physical Society of Japan, 78(12), p.123711_1 - 123711_4, 2009/12
Times Cited Count:41 Percentile:82.88(Physics, Multidisciplinary)Isobe, Mitsutaka*; Toi, Kazuo*; Matsushita, Hiroyuki*; Goto, Kazuyuki*; Suzuki, Chihiro*; Nagaoka, Kenichi*; Nakajima, Noriyoshi*; Yamamoto, Satoshi*; Murakami, Sadayoshi*; Shimizu, Akihiro*; et al.
Nuclear Fusion, 46(10), p.S918 - S925, 2006/10
Times Cited Count:31 Percentile:69.07(Physics, Fluids & Plasmas)no abstracts in English
Otsuki, Ryusei*; Nasu, Shoichi*; Matsushita, Masaki*; Fujii, Kimio; Ohashi, Kentaro*; Yamamoto, Ryoichi*
Funtai Oyobi Fummatsu Yakin, 51(8), p.633 - 634, 2004/08
We examined effects of deposition temperatures and collector radii onthe yields of fullerenes by arc discharge. Yields of fullerenes were 7-10%, 4-6% and 0.5-1% in the liquid nitrogen-cooling, water-cooling, non-cooling, respectively, and were better with a collector radius of 50mm than with that of 30mm. The weight ratios of C /C
/C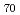 are about one for all runs of experiments.
are about one for all runs of experiments.
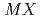 (
( =Pt, Pd;
=Pt, Pd; 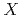 =P, As, Sb)
=P, As, Sb)Iwasaki, T.*; Sekiyama, Akira*; Yamasaki, Atsushi*; Okazaki, Makoto*; Kadono, Koji*; Utsunomiya, Hiroshi*; Imada, Shin*; Saito, Yuji; Muro, Takayuki*; Matsushita, Tomohiro*; et al.
Physical Review B, 65(19), p.195109_1 - 195109_9, 2002/05
Times Cited Count:24 Percentile:71.01(Materials Science, Multidisciplinary)no abstracts in English
Aoyama, Yoshio; Yamaguchi, Hiromi; Sakakibara, Tetsuro; Hanamoto, Yukio; Murata, Minoru*; Sasaki, Nao*; Nishikawa, Tsutomu*; Taniguchi, Shoji*; Shimazaki, Shinichi*; Park, J.*; et al.
no journal, ,
no abstracts in English
Aoyama, Yoshio; Yamaguchi, Hiromi; Sakakibara, Tetsuro; Hanamoto, Yukio; Murata, Minoru*; Sasaki, Nao*; Nishikawa, Tsutomu*; Taniguchi, Shoji*; Shimazaki, Shinichi*; Park, J.*; et al.
no journal, ,
no abstracts in English
Aoyama, Yoshio; Yamaguchi, Hiromi; Sakakibara, Tetsuro; Hanamoto, Yukio; Murata, Minoru*; Sasaki, Nao*; Nishikawa, Tsutomu*; Taniguchi, Shoji*; Shimazaki, Shinichi*; Park, J.*; et al.
no journal, ,
no abstracts in English
Yamaguchi, Hiromi; Miyamoto, Yasuaki; Sakakibara, Tetsuro; Hanamoto, Yukio; Aoyama, Yoshio; Sasaki, Nao*; Nishikawa, Tsutomu*; Murata, Minoru*; Muroi, Masayuki*; Park, J.*; et al.
no journal, ,
no abstracts in English
Tsujimoto, Yoshihiro*; Yamaura, Kazunari*; Matsushita, Yoshitaka*; Muromachi, Eiji*; Hayashi, Naoaki*; Kodama, Katsuaki; Igawa, Naoki; Shirako, Yuichi*; Akaogi, Masaki*
no journal, ,
Topotactic reaction of the n = 2 Ruddlesden-Popper phase Sr Fe
Fe O
O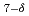 with the Teflon polymer yields a highly fluorinated phase Sr
with the Teflon polymer yields a highly fluorinated phase Sr Fe
Fe O
O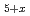 O
O (x
(x  0.44). The crystal/magnetic structures and anion composition are determined by a combination of neutron diffraction and
0.44). The crystal/magnetic structures and anion composition are determined by a combination of neutron diffraction and 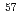 Fe M
Fe M ssbauer spectroscopy experiments. Fluoride anions selectively occupy the terminal apical anion sites, leading to the square pyramidal coordination around the Fe metal center. The oxyfluoride material adopts a simple antiferromagnetic ordered structure with k = (1/2 1/2 0) below 390 K.
ssbauer spectroscopy experiments. Fluoride anions selectively occupy the terminal apical anion sites, leading to the square pyramidal coordination around the Fe metal center. The oxyfluoride material adopts a simple antiferromagnetic ordered structure with k = (1/2 1/2 0) below 390 K.
Yamaguchi, Hiromi; Miyamoto, Yasuaki; Sakakibara, Tetsuro; Hanamoto, Yukio; Aoyama, Yoshio; Sasaki, Nao*; Nishikawa, Tsutomu*; Murata, Minoru*; Muroi, Masayuki*; Park, J.*; et al.
no journal, ,
no abstracts in English
Aoyama, Yoshio; Yamaguchi, Hiromi; Miyamoto, Yasuaki; Sakakibara, Tetsuro; Hanamoto, Yukio; Sasaki, Nao*; Nishikawa, Tsutomu*; Murata, Minoru*; Muroi, Masayuki*; Park, J.*; et al.
no journal, ,
no abstracts in English