Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kodaira, Takahide*; Ikeda, Ayumi*; Matsuyama, Emi*; Kono, Nobuho*; Oura, Kotone*; Sawada, Shinichi; Yamaki, Tetsuya; Nomura, Mikihiro*
no journal, ,
There has been a strong motivation to develop new cation exchange membranes suitable for the Bunsen reaction in the thermochemical water splitting IS process. We prepared cation exchange membranes by a radiation grafting polymerization method. The grafting reaction into a poly(ethylene-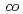 -tetrafluoroethylene) film was performed in a mixture of styrene and divinylbenzene (DVB). The membrane had an ion exchange capacity of 2.17 mmol g
-tetrafluoroethylene) film was performed in a mixture of styrene and divinylbenzene (DVB). The membrane had an ion exchange capacity of 2.17 mmol g while Nafion212 possessed 0.90 mmol g
while Nafion212 possessed 0.90 mmol g that is less than half of that for the grafted membrane. Both these membranes exhibited a similar water uptake, but the grafted membrane showed lower water permeation than Nafion212.
that is less than half of that for the grafted membrane. Both these membranes exhibited a similar water uptake, but the grafted membrane showed lower water permeation than Nafion212.
Kodaira, Takahide*; Ikeda, Ayumi*; Matsuyama, Emi*; Kono, Nobuho*; Oura, Kotone*; Sawada, Shinichi; Yamaki, Tetsuya; Nomura, Mikihiro*
no journal, ,
In the thermochemical water splitting IS process, the Bunsen reaction (SO + I
+ I + 2H
+ 2H O = H
O = H SO
SO + 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane, which renders separation procedures unnecessary. As part of a JST-ALCA project, therefore, we have been developing ion exchange membranes for this application by radiation-induced graft polymerization. The water permeation of the grafted membrane was lower than that of Nafion 212 under the condition of a similar water uptake.
+ 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane, which renders separation procedures unnecessary. As part of a JST-ALCA project, therefore, we have been developing ion exchange membranes for this application by radiation-induced graft polymerization. The water permeation of the grafted membrane was lower than that of Nafion 212 under the condition of a similar water uptake.
Kodaira, Takahide*; Ikeda, Ayumi*; Matsuyama, Emi*; Oura, Kotone*; Sawada, Shinichi; Yamaki, Tetsuya; Nomura, Mikihiro*
no journal, ,
There has been a strong motivation to develop new cation exchange membranes suitable for the Bunsen reaction in the thermochemical water splitting IS process. We prepared cation exchange membranes by a radiation grafting polymerization method. The grafting reaction into a poly(ethylene-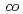 -tetrafluoroethylene) film was performed in a mixture of styrene and divinylbenzene (DVB). The grafted membrane showed two times lower water permeation flux and three times higher activation energy of water diffusion than Nafion212 though both the membranes exhibited a similar water uptake. Therefore, the DVB-based crosslinking in the graft polymer would restrict water permeation through the membrane.
-tetrafluoroethylene) film was performed in a mixture of styrene and divinylbenzene (DVB). The grafted membrane showed two times lower water permeation flux and three times higher activation energy of water diffusion than Nafion212 though both the membranes exhibited a similar water uptake. Therefore, the DVB-based crosslinking in the graft polymer would restrict water permeation through the membrane.
Ikeda, Ayumi*; Ono, Ryuhei*; Matsuyama, Emi*; Nomura, Mikihiro*; Tanaka, Nobuyuki; Kubo, Shinji
no journal, ,
In the IS process, the improvement of efficiency and the downsizing can be achieved if equilibrium inversion rate of HI decomposition is improved by using membrane reactor with hydrogen separation membrane. In this paper, the application to the silica hybrid membrane prepared by counter diffusion CVD method was investigated. As a result, we can obtain the H /HI permeation rate, 6820 and the H
/HI permeation rate, 6820 and the H permeance, 5.0
permeance, 5.0 10
10 mol m
mol m s
s Pa
Pa at 400
at 400  C, indicating that this membrane can be applied to this system. In addition, relation between SF
C, indicating that this membrane can be applied to this system. In addition, relation between SF and HI permeance of membrane was investigated and verified to show the positive correlation. This result indicates that HI permeance of membrane can be evaluated by using SF
and HI permeance of membrane was investigated and verified to show the positive correlation. This result indicates that HI permeance of membrane can be evaluated by using SF permeance, which can be easily treated.
permeance, which can be easily treated.
Kodaira, Takahide*; Oura, Kotone*; Ikeda, Ayumi*; Ono, Ryuhei*; Matsuyama, Emi*; Nomura, Mikihiro*; Sawada, Shinichi; Yamaki, Tetsuya; Tanaka, Nobuyuki; Kubo, Shinji
no journal, ,
Bunsen reactor in the IS process has a potential of the downsizing and the improvement of efficiency by using redox type reactor with an ion exchange membrane. The key to the performance of redox reactor is the development of the high performance ion exchange membrane. In this paper, we investigated the performance (proton transport number (t ) and water permeation factor (
) and water permeation factor ( )) of Nafion 212, which is the reference. As a result, t
)) of Nafion 212, which is the reference. As a result, t and
and  were 0.63 and 2.82, respectively, indicating that not only H
were 0.63 and 2.82, respectively, indicating that not only H but also I
but also I and water permeate through the membrane. The permeation of these components might cause the precipitation of sulfur and the rising of the voltage. Aftertime, we must new ion exchange membrane which can restrict the permeation of I
and water permeate through the membrane. The permeation of these components might cause the precipitation of sulfur and the rising of the voltage. Aftertime, we must new ion exchange membrane which can restrict the permeation of I and water.
and water.
Kodaira, Takahide*; Ikeda, Ayumi*; Matsuyama, Emi*; Kono, Nobuho*; Oura, Kotone*; Sawada, Shinichi; Yamaki, Tetsuya; Nomura, Mikihiro*
no journal, ,
In the thermochemical water splitting IS process, the Bunsen reaction (SO + I
+ I + 2H
+ 2H O = H
O = H SO
SO + 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane. As part of an ongoing JST-ALCA project, therefore, we developed ion exchange membranes for this reaction by radiation-induced graft polymerization, investigating their water permeation properties by the pervaporation method. Our membrane preparation involves the
+ 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane. As part of an ongoing JST-ALCA project, therefore, we developed ion exchange membranes for this reaction by radiation-induced graft polymerization, investigating their water permeation properties by the pervaporation method. Our membrane preparation involves the  -ray-induced grafting of styrene and divinylbenzene into poly(ethylene-
-ray-induced grafting of styrene and divinylbenzene into poly(ethylene-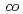 -tetrafluoroethylene) films and the subsequent sulfonation. Water permeate flux values at 25
-tetrafluoroethylene) films and the subsequent sulfonation. Water permeate flux values at 25 C were 7.4 and 19 kg/m
C were 7.4 and 19 kg/m h through the grafted membrane and Nafion 212 at the same water uptake (37%), respectively.
h through the grafted membrane and Nafion 212 at the same water uptake (37%), respectively.
Shiraishi, Junya; Honda, Mitsuru; Hayashi, Nobuhiko; Aiba, Nobuyuki; Toma, Mitsunori; Matsuyama, Akinobu; Naito, Osamu; Miyata, Yoshiaki; Inoue, Shizuo; Narita, Emi; et al.
no journal, ,
no abstracts in English
Kodaira, Takahide; Ikeda, Ayumi*; Matsuyama, Emi*; Kono, Nobuho*; Sawada, Shinichi; Yamaki, Tetsuya; Nomura, Mikihiro*
no journal, ,
In the thermochemical water splitting IS process, the Bunsen reaction (SO + I
+ I + 2H
+ 2H O = H
O = H SO
SO + 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane, which renders separation procedures unnecessary. As part of a JST-ALCA project, therefore, we have been developing ion exchange membranes for this application by using methods of radiation crosslinking and/or radiation graft polymerization. Our preliminary experiments made it possible to confirm controllability of the degree of grafting,
+ 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane, which renders separation procedures unnecessary. As part of a JST-ALCA project, therefore, we have been developing ion exchange membranes for this application by using methods of radiation crosslinking and/or radiation graft polymerization. Our preliminary experiments made it possible to confirm controllability of the degree of grafting, 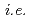 , ion exchange capacity by varying the conditions of the grafting.
, ion exchange capacity by varying the conditions of the grafting.
Kodaira, Takahide; Ikeda, Ayumi*; Matsuyama, Emi*; Kono, Nobuho*; Sawada, Shinichi; Yamaki, Tetsuya; Nomura, Mikihiro*
no journal, ,
In the thermochemical water splitting IS process, the Bunsen reaction (SO + I
+ I + 2H
+ 2H O = H
O = H SO
SO + 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane, which renders separation procedures unnecessary. As part of a JST-ALCA project, therefore, we have been developing ion exchange membranes for this application by using methods of radiation crosslinking and/or radiation graft polymerization. Our preliminary experiments made it possible to confirm controllability of the degree of grafting,
+ 2HI) needs to be achieved in an electrochemical cell with an ion exchange membrane, which renders separation procedures unnecessary. As part of a JST-ALCA project, therefore, we have been developing ion exchange membranes for this application by using methods of radiation crosslinking and/or radiation graft polymerization. Our preliminary experiments made it possible to confirm controllability of the degree of grafting, 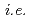 , ion exchange capacity by varying the conditions of the grafting. Importantly, controlling the fixed-charge density of the membrane should both lower permeability of SO
, ion exchange capacity by varying the conditions of the grafting. Importantly, controlling the fixed-charge density of the membrane should both lower permeability of SO and enhance the transport number of H
and enhance the transport number of H .
.
Kodaira, Takahide*; Ikeda, Ayumi*; Oura, Kotone*; Ono, Ryuhei*; Matsuyama, Emi*; Sawada, Shinichi; Yamaki, Tetsuya; Nomura, Mikihiro*
no journal, ,
no abstracts in English