Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Nagano, Tetsushi; Naganawa, Hirochika; Suzuki, Hideya; Toshimitsu, Masaaki*; Mitamura, Hisayoshi*; Yanase, Nobuyuki*; Grambow, B.
Analytical Sciences, 34(9), p.1099 - 1102, 2018/09
Times Cited Count:13 Percentile:43.38(Chemistry, Analytical)A previously reported emulsion flow (EF) extraction system does not include a device for refining used solvent. Therefore, the processing of large quantities of wastewater by using the EF extractor alone could lead to the accumulation of wastewater components into the solvent and diminished extraction performance. In the present study, we have developed a solvent-washing-type EF system, which is equipped with a unit for washing used solvent to prevent accumulation, and successfully applied it for treating uranium-containing wastewater.
Nagasu, Ryosuke*; Tanabe, Daijiro*; Yokotsuka, Satoshi*; Kumazawa, Noriyuki*; Ajiki, Takaya*; Aizawa, Yusuke*; Naganawa, Hirochika; Nagano, Tetsushi; Yanase, Nobuyuki*; Mitamura, Hisayoshi*; et al.
Kankyo Joka Gijutsu, 17(2), p.58 - 61, 2018/03
A new technology to suppress cesium migration from forests has been developed collaboratively by Ibaraki University, Kumagai-gumi Co., Ltd. and its group company, Technos, and JAEA. The new technology utilizes polyelectrolytes (polymers with electric charges) and clay minerals to control Cs migration with the aid of natural forces such as rainfall and rainwater runoff. In Imitate-mura, Fukushima, verification tests of the new technology have been performed and its effect on controlling Cs migration from forests to grass farm adjoining the forests has been proven.
Yamashita, Yuji*; Yanase, Nobuyuki; Nagano, Tetsushi; Mitamura, Hisayoshi; Naganawa, Hirochika
Journal of Radioanalytical and Nuclear Chemistry, 305(2), p.583 - 587, 2015/08
Times Cited Count:6 Percentile:42.50(Chemistry, Analytical)We propose a method for the decontamination and waste volume reduction of cesium-contaminated soil. The soils were solidified with an interpolyelectrolyte complex solution and classified into several size fractions by wet sieving.  -ray spectrometry of these fractions showed that the distribution ratio of the activity concentration of coarse soil particles decreased, whereas that of soil particles under 0.075 mm increased relative to reference samples. Results show that the fine soil particles, on which radioactive cesium accumulates, were removed from the surface of the coarse soil particles during, and remained in the washing water.
-ray spectrometry of these fractions showed that the distribution ratio of the activity concentration of coarse soil particles decreased, whereas that of soil particles under 0.075 mm increased relative to reference samples. Results show that the fine soil particles, on which radioactive cesium accumulates, were removed from the surface of the coarse soil particles during, and remained in the washing water.
Nagano, Tetsushi; Mitamura, Hisayoshi; Yanase, Nobuyuki; Naganawa, Hirochika; Yasuda, Kenichiro; Yamaguchi, Hiroaki*
Hoshasei Busshitsu No Kyuchaku, Josen Oyobi Taihoshasen Gjutsu Ni Okeru Zairyo, Seko, Sokutei No Shin Gijutsu, p.400 - 408, 2014/11
A method for monitoring radioactive cesium concentration in water using a cesium adsorption disk and a GM survey meter has been developed to ascertain whether the water quality meets standards on radiological contaminants in water. This method was successfully applied to monitoring of decontaminated water of an outdoor school swimming pool in Date City after Fukushima Daiichi Nuclear Power Plant accident.
Nagano, Tetsushi; Mitamura, Hisayoshi; Yamashita, Yuji; Yanase, Nobuyuki; Suzuki, Hideya; Naganawa, Hirochika
Solvent Extraction Research and Development, Japan, 21(1), p.111 - 117, 2014/00
Simulated electroless nickel plating liquid wastes have been processed by using an emulsion flow extractor of a counter current type with a special focus on influences of dilution of the liquid wastes on the extraction performance. The emulsion flow extractor provides an efficient liquid-liquid extraction by sending solutions without additional stirring or shaking. A solvent used in the present study was Shellsol D70 solution containing LIX84-I as an extractant for nickel and PC88A as an accelerating agent. As a result, it was found that increasing degree of dilution with water resulted in improvement of nickel extractabilities obtained from the emulsion flow experiments with a maximum value of 96% as well as those obtained from batch experiments. Droplet sizes at the lower and the upper sides of emulsion phases, estimated by using high-speed microscope, were 214  36
36  m and 415
m and 415  110
110  m, respectively.
m, respectively.
Nagano, Tetsushi; Yanase, Nobuyuki; Naganawa, Hirochika; Mitamura, Hisayoshi; Hanzawa, Yukiko; Mita, Yutaka; Kanda, Nobuhiro; Ohashi, Yusuke; Endo, Yuji; Matsubara, Tatsuo
Nihon Genshiryoku Gakkai Wabun Rombunshi, 12(4), p.277 - 285, 2013/12
no abstracts in English
Saegusa, Jun; Kurikami, Hiroshi; Yasuda, Ryo; Kurihara, Kazuo; Arai, Shigeki; Kuroki, Ryota; Matsuhashi, Shimpei; Ozawa, Takashi; Goto, Hiroaki; Takano, Takao; et al.
Health Physics, 104(3), p.243 - 250, 2013/03
Times Cited Count:3 Percentile:24.26(Environmental Sciences)After the Nuclear accident on March 2011, water discharge from many outdoor swimming pools in the Fukushima prefecture was suspended out of concern that radiocesium in the pool water would flow into farmlands. We have reviewed the existing flocculation method for decontaminating pool water and established a practical decontamination method by demonstrating the process at several pools in the Fukushima prefecture.
Nagano, Tetsushi; Mitamura, Hisayoshi; Yanase, Nobuyuki; Naganawa, Hirochika; Yasuda, Kenichiro; Yoshida, Zenko; Yamaguchi, Hiroaki*
Nihon Hoshasen Anzen Kanri Gakkai-Shi, 11(2), p.139 - 145, 2012/11
An on-site monitoring method for radioactive cesium concentration in water using a cesium adsorption disk and a GM survey meter has been developed to rapidly and easily ascertain whether the water quality meets standards on radiological contaminants in water. In this method, both dissolved and suspended forms of radioactive cesium are collected on the cesium adsorption disk by means of filtration of a water sample. Beta counting rate of the disk is converted into radioactivity using a conservative calibration factor obtained here. The present on-site method was applied to monitoring of decontaminated water of an outdoor school swimming pool in Date city after Fukushima Dai-ichi Nuclear Power Plant accident.
Naganawa, Hirochika; Kumazawa, Noriyuki*; Saito, Hiroshi*; Yanase, Nobuyuki; Mitamura, Hisayoshi; Nagano, Tetsushi; Kashima, Kaoru*; Fukuda, Tatsuya*; Yoshida, Zenko; Tanaka, Shunichi*
Nihon Genshiryoku Gakkai Wabun Rombunshi, 10(4), p.227 - 234, 2011/12
no abstracts in English
Shimojo, Kojiro; Mitamura, Hisayoshi; Mouri, Tsuyoshi; Naganawa, Hirochika
Chemistry Letters, 40(5), p.435 - 437, 2011/05
Times Cited Count:5 Percentile:23.75(Chemistry, Multidisciplinary)A simple fabrication strategy of DNA-transcribed silica materials has been developed. An ionic liquid incorporating an alkoxysilane group is capable of binding with DNA and acts as nuclei for subsequent sol-gel reaction with tetramethoxysilane. Unique silica materials with worm-like, rod-shaped, toroidal, or linearly fibrous structures are created from the same DNA template through the morphological transition of DNA induced by the ionic liquid.
Nagano, Tetsushi; Yanase, Nobuyuki; Hanzawa, Yukiko; Takada, Morio*; Mitamura, Hisayoshi; Sato, Tsutomu*; Naganawa, Hirochika
Water, Air, and Soil Pollution, 216(1-4), p.153 - 166, 2011/03
Times Cited Count:15 Percentile:39.28(Environmental Sciences)Schwertmannite is a poorly-crystalline ferric sulphate mineral that secondarily forms in acid mine drainages (AMD) as a result of the oxidative weathering of pyrite (FeS2), and is known to work as a naturally-occurring scavenger of some toxic elements in AMD-contaminated streams due to its high fixation potentials. In this study, to examine the feasibility of using schwertmannite in water purification technique, we evaluate the affinity of some selected elements to schwertmannite using two parameters: (1) conventional apparent solid-liquid partition coefficients between schwertmannite and stream waters, and (2) newly introduced parameters that correspond to ratios of ions fixed by schwertmannite to those existing as dissolved phases. As a result, both of the two parameters revealed that schwertmannite has high fixation potential for fluvial transport of various toxic anions such as V, Cr, As, Mo and Sb in AMD-contaminated streams, and that it could be used for purification of waters contaminated with these toxic anions.
Mitamura, Hisayoshi; Naganawa, Hirochika; Nagano, Tetsushi; Yanase, Nobuyuki; Hanzawa, Yukiko; Shimojo, Kojiro; Matsubara, Tatsuo; Mita, Yutaka; Taki, Tomihiro; Murata, Masato
JAEA-Research 2008-113, 27 Pages, 2009/03
An effective mass processing equipment using solvent extraction method, named "emulsion flow extractor," is the most promising apparatus for removal and recovery of uranium from liquid waste originated from decontamination of uranium-contaminated fluoride waste in the uranium conversion test facility and of used gas centrifuges in the uranium enrichment facility at Ningyo-toge environmental engineering center. Prior to application of the emulsion flow extractor for actual uranium-containing liquid waste, properties of some phosphorous extractants for extraction and separation of uranium and constituents from simulated liquid wastes were examined through batch tests. These preliminary tests revealed that D2EHPA would be a promising candidate for extractant used for treatment of the actual uranium-containing liquid wastes, and that the extractants with a surfactant like AOT would not be useful.
 -type montmorillonite; Potential chemical species of iron contaminant
-type montmorillonite; Potential chemical species of iron contaminantKozai, Naofumi; Inada, Koichi*; Adachi, Yoshifusa*; Kawamura, Sachi*; Kashimoto, Yusuke*; Kozaki, Tamotsu*; Sato, Seichi*; Onuki, Toshihiko; Sakai, Takuro; Sato, Takahiro; et al.
Journal of Solid State Chemistry, 180(8), p.2279 - 2289, 2007/08
Times Cited Count:14 Percentile:47.18(Chemistry, Inorganic & Nuclear)Fe -montmorillonite with Fe
-montmorillonite with Fe ions occupying cation exchange sites is an ideal transformation product in bentonite buffer material. We previously prepared a Fe
ions occupying cation exchange sites is an ideal transformation product in bentonite buffer material. We previously prepared a Fe -montmorillonite sample using a FeCl
-montmorillonite sample using a FeCl solution under an inert gas condition. This study attempted to determine the potential contaminant iron chemical species in the sample. It was found that a small amount of Cl
solution under an inert gas condition. This study attempted to determine the potential contaminant iron chemical species in the sample. It was found that a small amount of Cl ions remained dispersed throughout the sample. The Cl
ions remained dispersed throughout the sample. The Cl ion retention may be due to the adsorption of FeCl
ion retention may be due to the adsorption of FeCl in the initial FeCl
in the initial FeCl solution and the subsequent containment of the Cl
solution and the subsequent containment of the Cl ions that are dissociated from the FeCl
ions that are dissociated from the FeCl during excess salt removal treatment. The latter may be explained by the slow release of the remaining Cl
during excess salt removal treatment. The latter may be explained by the slow release of the remaining Cl ions from the collapsed interlayer of the montmorillonite or the transformation of a minor fraction of the remaining FeCl
ions from the collapsed interlayer of the montmorillonite or the transformation of a minor fraction of the remaining FeCl to iron (III) hydroxide chloride complexes having low solubility.
to iron (III) hydroxide chloride complexes having low solubility.
Naganawa, Hirochika; Shimojo, Kojiro; Mitamura, Hisayoshi; Sugo, Yumi; Noro, Junji*; Goto, Masahiro*
Solvent Extraction Research and Development, Japan, 14, p.151 - 159, 2007/00
A compound having diglycol amic acid frame, DODGAA, has been synthesized as a new "green" extractant for lanthanides. The new extractant composed, hydrogen, oxygen, and nitrogen atoms is fully combustible to gases without solid waste and its solubility in water is found to be very low. These properties enable us to avoid the burden of industrial waste produced by the incineration of deteriorated extractant and the risk of aquatic environmental pollution. In the extraction ability for lanthanide ions and the ability for their mutual separation, DODGAA is much superior to carboxykic acid type extractants, such as Versatic10, that can also be completely combustible. The extraction and separation performance of DODGAA is comparable to that of organophosphorus extractants, such as PC-88A and DEHPA, that are widely used in current industry but less "green".
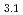 (OCOCH
(OCOCH )
)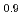
 0.9H
0.9H O
OKozai, Naofumi; Mitamura, Hisayoshi; Fukuyama, Hiroyasu; Esaka, Fumitaka; Komarneni, S.*
Microporous and Mesoporous Materials, 89(1-3), p.123 - 131, 2006/03
Times Cited Count:12 Percentile:40.72(Chemistry, Applied)Layered transition metal hydroxide salt (LTMHS) is a group of anion-exchangeable layered compounds. Although LTMHSs have recentely attracted attention of researches on anion exchange and intercalation, very limited numbers of reports have been published on their synthesis, characteristics, and applications. This paper describes basic characteristics of a new LTMHS, nickel-copper hydroxide acetate. Hydrothermal Heating of an aqueous solution containing nickel acetate, copper acetate, and hydrogen peroxide to 150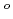 C for 4h yielded a layered compound with an analytical composition of NiCu(OH)
C for 4h yielded a layered compound with an analytical composition of NiCu(OH)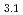 (OCOCH
(OCOCH )
)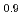 0.9H
0.9H O. This compound does not take up Cl
O. This compound does not take up Cl and NO
and NO
 in aqueous solution but takes up multivalent anions and shows high selectivity in uptake of toxic SeO
in aqueous solution but takes up multivalent anions and shows high selectivity in uptake of toxic SeO
 and AsO
and AsO
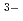 . This compound may find applicarion in the removal of those toxic anions form natural water and wastewater rich in Cl
. This compound may find applicarion in the removal of those toxic anions form natural water and wastewater rich in Cl and NO
and NO
 .
.
Kozai, Naofumi; Mitamura, Hisayoshi; Fukuyama, Hiroyasu; Esaka, Fumitaka; Komarneni, S.*
Journal of Materials Research, 20(11), p.2997 - 3003, 2005/11
Layered transition metal hydroxide salt (LTMHS) is a group of anion-exchangeable layered compounds. LTMHSs have lately attraced attention of researchs on anion exchange and intercalation but very limited numbers of reports have been published on their synthesis, characteristics, and application. This study reports basic properties of a layered copper hyroxide acetate synthesized by a method modified from that of the previous studies. Titration of copper acetate solution with a dilute NaOH solution to pH 6.5 and subsequent aging at 313 K yielded a layered copper hydroxide acetate. This compound has some properties similar to those of the previously known copper hydroxide acetate, Cu (OH)
(OH) (OCOCH
(OCOCH )H
)H O. The present copper hydroxide acetate is dissimilar to the previous compound in morphology, stability of bonding between the interlayer acetate ions and the matrix hydroxides, and reaction with anions in aqueous solutions.
O. The present copper hydroxide acetate is dissimilar to the previous compound in morphology, stability of bonding between the interlayer acetate ions and the matrix hydroxides, and reaction with anions in aqueous solutions.
Kozai, Naofumi; Mitamura, Hisayoshi; Onuki, Toshihiko; Sakai, Takuro; Sato, Takahiro; Oikawa, Masakazu*
Nuclear Instruments and Methods in Physics Research B, 231(1-4), p.530 - 535, 2005/04
Times Cited Count:9 Percentile:55.13(Instruments & Instrumentation)Applicability of micro-PIXE analysis to quantitative evaluation of heavy elements sorbed on minerals was investigated to get better understanding of sorption and distribution of heavy elements onto mixture of minerals in soil. For this, external standards, that is, heavy element-sorbing minerals of uniform shape and size, were analyzed by micro-PIXE. It was found that such external standards were available to quantitative evaluation by micro-PIXE though their applicability may be limited.
Mitamura, Hisayoshi
JAERI-Review 2003-007, 54 Pages, 2003/03
The inevitable increases of food production and energy consumption with an increase in world population become main causes of an increase of nitrate load to the environment. Although nitrogen is essential for the growth of animal and plant as a constituent element of protein, excessive nitrate load to the environment contaminates groundwater resources used as drinking water and leads to seriously adverse effects on the health of man and livestock. In order to clarify the problem of nitrate contamination of groundwater and search a new trend of technology development from the viewpoint of environmental remediation and protection, the present paper has reviewed adverse effects of nitrate on human health, the actual state of nitrogen cycle, several kinds of nitrate sources, treatment methods for reducing nitrate level, etc.
 and Na
and Na O additives on properties synthetic rock of fly ash from municipal waste incinerator
O additives on properties synthetic rock of fly ash from municipal waste incineratorMitamura, Hisayoshi; Bamba, Tsunetaka; Maeda, Toshikatsu
Nihon Seramikkusu Kyokai Gakujutsu Rombunshi, 110(1277), p.55 - 59, 2002/01
Times Cited Count:1 Percentile:19.02(Materials Science, Ceramics)Influence of additives and hot-pressing temperature on properties of the synthetic rock was investigated, which was developed for making harmless and stabilizing fly ash from municipal waste incinerator. Sintering test at 1200 C for 16 h revealed that addition of 20 and 1.5 wt% of TiO
C for 16 h revealed that addition of 20 and 1.5 wt% of TiO and Na
and Na O, respectively, is necessary for preparation of dense products. X-ray diffractometry showed that these additives promoted the formation of perovskite (CaTiO
O, respectively, is necessary for preparation of dense products. X-ray diffractometry showed that these additives promoted the formation of perovskite (CaTiO ) and gehlenite (Ca
) and gehlenite (Ca Al
Al SiO
SiO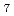 ) minerals. During hot pressing, bulk density of the synthetic rock increased steeply with temperature between 1000 and 1100
) minerals. During hot pressing, bulk density of the synthetic rock increased steeply with temperature between 1000 and 1100 C. On the other hand, its open porosity decreased rapidly with temperature between 1050 and 1150
C. On the other hand, its open porosity decreased rapidly with temperature between 1050 and 1150 C. These facts imply that the temperature of 1100
C. These facts imply that the temperature of 1100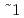 150
150 C is practical in hot pressing of fly ash.
C is practical in hot pressing of fly ash.
Ishiyama, Takashi; Bamba, Tsunetaka; Mitamura, Hisayoshi; Maeda, Toshikatsu
Haikibutsu Gakkai Rombunshi, 12(2), p.82 - 86, 2001/03
no abstracts in English