Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kataoka, Takahiro*; Ishida, Tsuyoshi*; Naoe, Shota*; Kanzaki, Norie; Sakoda, Akihiro; Tanaka, Hiroshi; Mitsunobu, Fumihiro*; Yamaoka, Kiyonori*
Journal of Radiation Research (Internet), 63(5), p.719 - 729, 2022/09
Times Cited Count:2 Percentile:47.19(Biology)Sakoda, Akihiro; Ishida, Tsuyoshi*; Kanzaki, Norie; Tanaka, Hiroshi; Kataoka, Takahiro*; Mitsunobu, Fumihiro*; Yamaoka, Kiyonori*
International Journal of Environmental Research and Public Health, 19(13), p.7761_1 - 7761_12, 2022/07
Times Cited Count:0 Percentile:0(Environmental Sciences)In specific situations such as bathing in a radon spa, where the radon activity concentration in thermal water is far higher than that in air, it has been revealed that radon uptake via skin can occur and should be considered for more precise dose evaluation. The primary aim of the present study was to numerically demonstrate the distribution as well as the degree of diffusion of radon in the skin, with a focus on its surface layers (i.e., stratum corneum). We made a biokinetic model that included diffusion theory at the stratum corneum, and measured radon solubility in the stratum corneum to get a crucial parameter. The implementation of the model suggested that the diffusion coefficient in the stratum corneum was as low as general radon-proof sheets. The depth profile of radon in the skin was found to be that after a 20-minute immersion in water, the radon activity concentration at the top surface skin layer was approximately 1000 times higher than that at the viable skin layer. The information on the position of radon as a radiation source would contribute to special dose evaluation where specific target cell layers are assumed for the skin.
Kataoka, Takahiro*; Naoe, Shota*; Murakami, Kaito*; Yukimine, Ryohei*; Fujimoto, Yuki*; Kanzaki, Norie; Sakoda, Akihiro; Mitsunobu, Fumihiro*; Yamaoka, Kiyonori*
Journal of Clinical Biochemistry and Nutrition, 70(2), p.154 - 159, 2022/03
Times Cited Count:3 Percentile:57.52(Nutrition & Dietetics)Kataoka, Takahiro*; Shuto, Hina*; Naoe, Shota*; Yano, Junki*; Kanzaki, Norie; Sakoda, Akihiro; Tanaka, Hiroshi; Hanamoto, Katsumi*; Mitsunobu, Fumihiro*; Terato, Hiroaki*; et al.
Journal of Radiation Research (Internet), 62(5), p.861 - 867, 2021/09
Times Cited Count:5 Percentile:55.27(Biology)Sakoda, Akihiro; Ishimori, Yuu; Kanzaki, Norie; Tanaka, Hiroshi; Kataoka, Takahiro*; Mitsunobu, Fumihiro*; Yamaoka, Kiyonori*
Journal of Radiation Research (Internet), 62(4), p.634 - 644, 2021/07
Times Cited Count:3 Percentile:38.06(Biology)It is held that the skin dose from radon progeny is not negligibly small and that introducing cancer is a possible consequence under normal circumstances, while there are a number of uncertainties in terms of related parameters such as activity concentrations in air, target cells in skin, skin covering materials, and deposition velocities. Meanwhile, an interesting proposal emerged in that skin exposure to natural radon-rich thermal water as part of balneotherapy can produce an immune response to induce beneficial health effects. The goal of the present study was to obtain generic dose coefficients with a focus on the radon progeny deposited on the skin in air or water in relation to risk or therapeutic assessments. We thus first estimated the skin deposition velocities of radon progeny in the two media based on data from the latest human studies. Using the optimized velocities, skin dosimetry was then performed under different assumptions regarding alpha-emitting source position and target cell (i.e., basal cells or Langerhans cells). Furthermore, the impact of the radon progeny deposition on effective doses from all exposure pathways relating to "radon exposure" was assessed using various possible scenarios. It was found that in both exposure media, effective doses from radon progeny inhalation are one to four orders of magnitude higher than those from the other pathways. In addition, absorbed doses on the skin can be the highest among all pathways when the radon activity concentrations in water are two or more orders of magnitude higher than those in air.
Kataoka, Takahiro*; Kanzaki, Norie; Sakoda, Akihiro; Shuto, Hina*; Yano, Junki*; Naoe, Shota*; Tanaka, Hiroshi; Hanamoto, Katsumi*; Terato, Hiroaki*; Mitsunobu, Fumihiro*; et al.
Journal of Radiation Research (Internet), 62(2), p.206 - 216, 2021/03
Times Cited Count:6 Percentile:61.83(Biology)Radon inhalation activates antioxidative functions in mouse organs, thereby contributing to inhibition of oxidative stress-induced damage. Therefore, in this study, we evaluated the redox state of various organs in mice following radon inhalation. Mice inhaled radon at concentrations of 2 or 20 kBq/m for 1, 3, or 10 days. The relationship between antioxidative function and oxidative stress was evaluated by principal component analysis (PCA) and correlation coefficient compared with control mice subjected to sham inhalation. These findings suggested that radon inhalation altered the redox state in organs, but that the characteristics varied depending on the redox state in organs.
for 1, 3, or 10 days. The relationship between antioxidative function and oxidative stress was evaluated by principal component analysis (PCA) and correlation coefficient compared with control mice subjected to sham inhalation. These findings suggested that radon inhalation altered the redox state in organs, but that the characteristics varied depending on the redox state in organs.
Kobashi, Yusuke*; Kataoka, Takahiro*; Kanzaki, Norie; Ishida, Tsuyoshi*; Sakoda, Akihiro; Tanaka, Hiroshi; Ishimori, Yuu; Mitsunobu, Fumihiro*; Yamaoka, Kiyonori*
Radiation and Environmental Biophysics, 59(3), p.473 - 482, 2020/08
Times Cited Count:5 Percentile:40.94(Biology)Radon therapy has been traditionally performed globally for oxidative stress-related diseases. Many researchers have studied the beneficial effects of radon exposure in living organisms. However, the effects of thoron, a radioisotope of radon, have not been fully examined. In this study, we aimed to compare the biological effects of radon and thoron inhalation on mouse organs with a focus on oxidative stress. Male BALB/c mice were randomly divided into 15 groups: sham inhalation, radon inhalation at a dose of 500 Bq/m or 2000 Bq/m
or 2000 Bq/m , and thoron inhalation at a dose of 500 Bq/m
, and thoron inhalation at a dose of 500 Bq/m or 2000 Bq/m
or 2000 Bq/m were carried out. Immediately after inhalation, mouse tissues were excised for biochemical assays. The results showed a significant increase in superoxide dismutase and total glutathione, and a significant decrease in lipid peroxide following thoron inhalation under several conditions. Additionally, similar effects were observed for different doses and inhalation times between radon and thoron. Our results suggest that thoron inhalation also exerts antioxidative effects against oxidative stress in organs. However, the inhalation conditions should be carefully analyzed because of the differences in physical characteristics between radon and thoron.
were carried out. Immediately after inhalation, mouse tissues were excised for biochemical assays. The results showed a significant increase in superoxide dismutase and total glutathione, and a significant decrease in lipid peroxide following thoron inhalation under several conditions. Additionally, similar effects were observed for different doses and inhalation times between radon and thoron. Our results suggest that thoron inhalation also exerts antioxidative effects against oxidative stress in organs. However, the inhalation conditions should be carefully analyzed because of the differences in physical characteristics between radon and thoron.
Etani, Reo*; Kataoka, Takahiro*; Kanzaki, Norie*; Sakoda, Akihiro; Tanaka, Hiroshi; Ishimori, Yuu; Mitsunobu, Fumihiro*; Taguchi, Takehito*; Yamaoka, Kiyonori*
Journal of Radiation Research, 58(5), p.614 - 625, 2017/05
Times Cited Count:13 Percentile:53.9(Biology)Radon therapy using radon (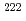 Rn) gas is classified into two types of treatment: inhalation of radon gas and drinking water containing radon. Although short- or long-term intake of spa water is effective in increasing gastric mucosal blood flow, and spa water therapy is useful for treating chronic gastritis and gastric ulcer, the underlying mechanisms for and precise effects of radon protection against mucosal injury are unclear. In the present study, we examined the protective effects of hot spring water drinking and radon inhalation on ethanol-induced gastric mucosal injury in mice. Mice inhaled radon at a concentration of 2000 Be/m
Rn) gas is classified into two types of treatment: inhalation of radon gas and drinking water containing radon. Although short- or long-term intake of spa water is effective in increasing gastric mucosal blood flow, and spa water therapy is useful for treating chronic gastritis and gastric ulcer, the underlying mechanisms for and precise effects of radon protection against mucosal injury are unclear. In the present study, we examined the protective effects of hot spring water drinking and radon inhalation on ethanol-induced gastric mucosal injury in mice. Mice inhaled radon at a concentration of 2000 Be/m for 24 h or were provided with hot spring water for 2 weeks. The activity density of
for 24 h or were provided with hot spring water for 2 weeks. The activity density of 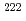 Rn ranged from 663 Bq/l (start point of supplying) to 100 Bq/l (end point of supplying).Mice were then orally administered ethanol at three concentrations. The ulcer index (UI), an indicator of mucosal injury, increased in response to the administration of ethanol; however, treatment with either radon inhalation or hot spring water inhibited the elevation in the UI due to ethanol. Although no significant differences in antioxidative enzymes were observed between the radon-treated groups and the non-treated control groups, lipid peroxide levels were significantly lower in the stomachs of mice pre-treated with radon or hot spring water. These results suggest that hot spring water drinking and radon inhalation inhibit ethanol-induced gastric mucosal injury.
Rn ranged from 663 Bq/l (start point of supplying) to 100 Bq/l (end point of supplying).Mice were then orally administered ethanol at three concentrations. The ulcer index (UI), an indicator of mucosal injury, increased in response to the administration of ethanol; however, treatment with either radon inhalation or hot spring water inhibited the elevation in the UI due to ethanol. Although no significant differences in antioxidative enzymes were observed between the radon-treated groups and the non-treated control groups, lipid peroxide levels were significantly lower in the stomachs of mice pre-treated with radon or hot spring water. These results suggest that hot spring water drinking and radon inhalation inhibit ethanol-induced gastric mucosal injury.
Ishimori, Yuu; Tanaka, Hiroshi; Sakoda, Akihiro; Kataoka, Takahiro*; Yamaoka, Kiyonori*; Mitsunobu, Fumihiro*
Radiation and Environmental Biophysics, 56(2), p.161 - 165, 2017/05
Times Cited Count:7 Percentile:38.06(Biology)In order to investigate the biokinetics of inhaled radon, radon concentrations in mouse tissues and organs were determined after mice had been exposed to about 1 MBq/m of radon in air. Radon concentrations in mouse blood and in other tissues and organs were measured with a liquid scintillation counter and with a well-type HP Ge detector, respectively. Radon concentration in mouse blood was 0.410
of radon in air. Radon concentrations in mouse blood and in other tissues and organs were measured with a liquid scintillation counter and with a well-type HP Ge detector, respectively. Radon concentration in mouse blood was 0.410 0.016 Bq/g when saturated with 1 MBq/m
0.016 Bq/g when saturated with 1 MBq/m of radon concentration in air. In addition, average partition coefficients obtained were 0.74
of radon concentration in air. In addition, average partition coefficients obtained were 0.74 0.19 for liver, 0.46
0.19 for liver, 0.46 0.13 for muscle, 9.09
0.13 for muscle, 9.09 0.49 for adipose tissue, and 0.22
0.49 for adipose tissue, and 0.22 0.04 for other organs. With these results, a value of 0.414 for the blood-to-air partition coefficient was calculated by means of our physiologically based pharmacokinetic model. The time variation of radon concentration in mouse blood during exposure to radon was also calculated. All results are compared in detail with those found in the literature.
0.04 for other organs. With these results, a value of 0.414 for the blood-to-air partition coefficient was calculated by means of our physiologically based pharmacokinetic model. The time variation of radon concentration in mouse blood during exposure to radon was also calculated. All results are compared in detail with those found in the literature.
Etani, Reo*; Kataoka, Takahiro*; Kanzaki, Norie*; Sakoda, Akihiro; Tanaka, Hiroshi; Ishimori, Yuu; Mitsunobu, Fumihiro*; Yamaoka, Kiyonori*
Journal of Radiation Research, 57(3), p.250 - 257, 2016/06
Times Cited Count:10 Percentile:46.3(Biology)Although radon therapy is indicated for hyperuricemia, the underlying mechanisms of action have not yet been elucidated in detail. Therefore, we herein examined the inhibitory effects of radon inhalation and hot spring water drinking on potassium oxonate (PO)-induced hyperuricemia in mice. After mice inhaled radon at a concentration of 2000 Bq/m for 24 h or were given hot spring water for 2 weeks, they were administrated PO. Radon inhalation or hot spring water drinking significantly inhibited elevations in serum uric acid levels through the suppression of xanthine oxidase activity in the liver. Radon inhalation activated anti-oxidative functions in the liver and kidney. These results suggest that radon inhalation inhibits PO-induced hyperuricemia by activating anti-oxidative functions, while hot spring water drinking may suppress PO-induced elevations in serum uric acid levels through the pharmacological effects of the chemical compositions dissolved in it.
for 24 h or were given hot spring water for 2 weeks, they were administrated PO. Radon inhalation or hot spring water drinking significantly inhibited elevations in serum uric acid levels through the suppression of xanthine oxidase activity in the liver. Radon inhalation activated anti-oxidative functions in the liver and kidney. These results suggest that radon inhalation inhibits PO-induced hyperuricemia by activating anti-oxidative functions, while hot spring water drinking may suppress PO-induced elevations in serum uric acid levels through the pharmacological effects of the chemical compositions dissolved in it.
Ishimori, Yuu; Sakoda, Akihiro; Tanaka, Hiroshi; Mitsunobu, Fumihiro*; Yamaoka, Kiyonori*; Kataoka, Takahiro*; Etani, Reo*
JAEA-Research 2015-024, 41 Pages, 2016/03
Okayama University and the Japan Atomic Energy Agency (JAEA) have carried out the collaborative study of physiological effects of inhaled radon for the low-dose range. Main assignments were as follows. Based on the clinical knowledge, Misasa Medical Center (Okayama University Hospital) clarified the issues that should be addressed. Graduate School of Health Sciences (Okayama University) supervised the research and studied the biological responses. The JAEA made the development and control of a facility for radon inhalation experiments and the investigation of biokinetics and exposure doses of radon. From 2009 to 2013, the following results were obtained. (1) Literature on drinking effects of radon hot spring water was surveyed to determine the present tasks. (2) Under the present experimental conditions, drinking of hot spring water into which radon was intentionally introduced using the equipment in the facility did not have significant effects on mice. (3) Inhibitory effects of antioxidant pre-supplements (Vitamins C and E) and radon pre-inhalation on hepatic or renal oxidative damage were examined to make the comparison. (4) In order to discuss biological responses quantitatively following radon inhalation, the biokinetics of inhaled radon were studied. (5) Some exposure routes due to inhalation of radon or its progeny were modeled to calculate organ doses in mice.
Kataoka, Takahiro*; Sakoda, Akihiro; Etani, Reo*; Ishimori, Yuu; Mitsunobu, Fumihiro*; Yamaoka, Kiyonori*
Onsen Kagaku, 64(4), p.380 - 387, 2015/03
Radon therapy using radon hot spring has been performed at Misasa Medical Center, Okayama University Hospital. This therapy can relieve some symptoms like pain. There have been many clinical studies, but very little data available to explain why radon inhalation results in such positive effects. The present paper mainly mentions our recent studies on health effects of radon hot spring. To clarify the radon effects, we first developed a radon exposure system for small animals. Using this system, especially in terms of antioxidative functions, we have examined effects of radon inhalation on mice. One of the results showed that the inhalation increased the activity of superoxide dismutase, which is an antioxidative enzyme, in many organs. The protective effect of radon on type I diabetes in mice was also shown. These findings indicate that the activation of antioxidative functions induced by radon inhalation relieves the symptoms brought by reactive oxygen species.
Sakoda, Akihiro; Ishimori, Yuu; Yamaoka, Kiyonori*; Kataoka, Takahiro*; Mitsunobu, Fumihiro*
Radiation and Environmental Biophysics, 52(3), p.389 - 395, 2013/08
Times Cited Count:11 Percentile:45.17(Biology)This paper provides absorbed doses arising from radon gas in air retained in lung airway lumens. Because radongas exposure experiments often use small animals, the calculation was performed for mice and rats. For reference, the corresponding computations were also done for humans. Assuming that radon concentration in airway lumens is the same as that in the environment, its progeny's production in and clearance from airways were simulated. Absorbed dose rates were obtained for three lung regions and the whole lung, considering that secretory and basal cells are sensitive to radiation. The results showed that absorbed dose rates for all lung regions and whole lung increase from mice to rats to humans. For example, the dose rates for the whole lung were 25.4 in mice, 41.7 in rats, and 59.9 pGy/(Bq/m )/h in humans. Furthermore, these values were also compared with lung dose rates from two other types of exposures, i.e., due to inhalation of radon or its progeny, which were already reported. It was confirmed that the direct inhalation of radon progeny in the natural environment, which is known as a cause of lung cancer, results in the highest dose rates for all species. Based on the present calculations, absorbed dose rates of the whole lung from radon gas were lower by a factor of about 550 (mice), 200 (rats) or 70 (humans) than those from radon progeny inhalation. The calculated dose rate values are comparatively small. Nevertheless, the present study is considered to contribute to our understanding of doses from inhalation of radon and its progeny.
)/h in humans. Furthermore, these values were also compared with lung dose rates from two other types of exposures, i.e., due to inhalation of radon or its progeny, which were already reported. It was confirmed that the direct inhalation of radon progeny in the natural environment, which is known as a cause of lung cancer, results in the highest dose rates for all species. Based on the present calculations, absorbed dose rates of the whole lung from radon gas were lower by a factor of about 550 (mice), 200 (rats) or 70 (humans) than those from radon progeny inhalation. The calculated dose rate values are comparatively small. Nevertheless, the present study is considered to contribute to our understanding of doses from inhalation of radon and its progeny.
Ishimori, Yuu; Sakoda, Akihiro; Tanaka, Hiroshi; Mitsunobu, Fumihiro*; Yamaoka, Kiyonori*; Kataoka, Takahiro*; Yamato, Keiko*; Nishiyama, Yuichi*
JAEA-Research 2013-005, 60 Pages, 2013/06
Okayama University and the Japan Atomic Energy Agency (JAEA) have carried out the collaborative study of physiological effects of inhaled radon for the low-dose range. From 2007 to 2011, the following results were obtained. (1) Literature on effects of radon for the low-dose range was surveyed to determine the present tasks. (2) The first Japanese large-scale facility was developed for radon inhalation experiments with small animals. (3) Relationships between radon concentration and inhalation time were widely examined to understand the change in antioxidative functions due to radon, which are the most basic parameters. (4) Inhibitory effects of radon on oxidative damages were observed using model mice with reactive oxygen- or free radical-related diseases like alcohol-induced oxidative damages and type I diabetes. (5) In order to discuss biological responses quantitatively following radon inhalation, the biokinetics of inhaled radon was examined and the model for calculation of absorbed doses for organs and tissues was obtained.
Sakoda, Akihiro; Ishimori, Yuu; Fukao, Kosuke*; Yamaoka, Kiyonori*; Kataoka, Takahiro*; Mitsunobu, Fumihiro*
Radiation and Environmental Biophysics, 51(4), p.425 - 442, 2012/11
Times Cited Count:14 Percentile:55.77(Biology)Biological response of exposure to radon progeny has long been investigated, but there are few studies in which absorbed doses in lungs were estimated if laboratory animals were used. The present study is the first attempt to calculate the doses of inhaled radon progeny for mice. For reference, the doses for rats and humans were also computed with the corresponding models. Lung deposition of particles, their clearance, and energy deposition of alpha particles to sensitive tissues were systematically simulated. Absorbed doses to trachea and bronchi (BB), bronchioles and terminal bronchioles (bb), alveolar-interstitial (AI) regions, and whole lung were first provided as a function of monodisperse radon-progeny particles with an equilibrium equivalent radon concentration of 1 Bq m-3 (equilibrium factor: 0.4 and unattached fraction: 0.01). Based on the results, absorbed doses were then calculated for (1) a reference mine condition and (2) a condition previously used for animal experiments. It was found that the whole lung doses for mice, rats and humans were 34.8, 20.7 and 10.7 nGy (Bq m )
) h
h for the mine condition, respectively, while they were 16.9, 9.9 and 6.5 nGy (Bq m
for the mine condition, respectively, while they were 16.9, 9.9 and 6.5 nGy (Bq m )
) h
h for the animal experimental condition. In both cases, the values of mice are about 2 times higher than those of rats, and about 3 times higher than those of humans. Comparison of our data on rats and humans with those published in the literature shows an acceptable agreement, suggesting the validity of the present modeling for mice. In the future, a more sophisticated dosimetric study of inhaled radon progeny in mice would be desirable to demonstrate how anatomical, physiological and environmental parameters can influence absorbed doses.
for the animal experimental condition. In both cases, the values of mice are about 2 times higher than those of rats, and about 3 times higher than those of humans. Comparison of our data on rats and humans with those published in the literature shows an acceptable agreement, suggesting the validity of the present modeling for mice. In the future, a more sophisticated dosimetric study of inhaled radon progeny in mice would be desirable to demonstrate how anatomical, physiological and environmental parameters can influence absorbed doses.
Aoyama, Yutaka*; Kataoka, Takahiro*; Nakagawa, Shinya*; Sakoda, Akihiro*; Ishimori, Yuu; Mitsunobu, Fumihiro*; Yamaoka, Kiyonori*
Iranian Journal of Radiation Research, 9(4), p.221 - 229, 2012/03
The aim of this study was to analyze the effects of thoron and thermal treatment for aging-related diseases in humans. All subjects inhaled thoron with a high concentration (about 4900 Bq/m ) for 2 weeks. Blood pressures were measured and blood samples were collected after each treatment 1, 2 and 3 weeks after the first treatment. The
) for 2 weeks. Blood pressures were measured and blood samples were collected after each treatment 1, 2 and 3 weeks after the first treatment. The  -atrial natriuretic peptide level of the rheumatoid arthritis group was increased and the blood pressure was significantly decreased. Superoxide dismutase activity of rheumatoid arthritis group was significantly increased by treatment. In addition, thoron and thermal treatment significantly enhanced the concanavalin A-induced mitogen response and increased the level of CD4-positive cells; it decreased the level of CD8-positive cells. The results suggest that thoron and thermal treatment activates antioxidative function. Furthermore, these findings suggest that thoron and thermal treatment prevents diabetic ketoacidosis and contributes to the prevention of aging-related diseases. Thoron and thermal therapy may be part of the mechanism for the alleviation of diabetes mellitus and rheumatoid arthritis.
-atrial natriuretic peptide level of the rheumatoid arthritis group was increased and the blood pressure was significantly decreased. Superoxide dismutase activity of rheumatoid arthritis group was significantly increased by treatment. In addition, thoron and thermal treatment significantly enhanced the concanavalin A-induced mitogen response and increased the level of CD4-positive cells; it decreased the level of CD8-positive cells. The results suggest that thoron and thermal treatment activates antioxidative function. Furthermore, these findings suggest that thoron and thermal treatment prevents diabetic ketoacidosis and contributes to the prevention of aging-related diseases. Thoron and thermal therapy may be part of the mechanism for the alleviation of diabetes mellitus and rheumatoid arthritis.
Kataoka, Takahiro*; Sakoda, Akihiro*; Yoshimoto, Masaaki*; Toyota, Teruaki*; Yamamoto, Yuki*; Ishimori, Yuu; Hanamoto, Katsumi*; Kawabe, Atsushi*; Mitsunobu, Fumihiro*; Yamaoka, Kiyonori*
Radiation Safety Management, 10(1), p.1 - 7, 2011/12
We examined the effect of continuous radon inhalation on acute alcohol-induced oxidative damage of mouse liver and brain. Assay of antioxidative functions indicated that lipid peroxide levels in both the liver and brain of the alcohol-treated mice were significantly higher than those of the saline-treated mice. However, the lipid peroxide level in the liver, but not in the brain, of alcohol-treated mice was significantly decreased by radon inhalation whereas that in the brain of saline-treated mice, but not in the liver of saline-treated mice, was significantly increased by radon inhalation. These findings suggest that radon inhalation inhibits alcohol-induced oxidative damage of liver due to activation of antioxidative functions and that radon inhalation exert only a week effect on the brains in comparing with the livers. They further suggest that alcohol administration protects against oxidative damage of the brain that is induced by radon inhalation.
Kataoka, Takahiro*; Sakoda, Akihiro; Ishimori, Yuu; Toyota, Teruaki*; Nishiyama, Yuichi*; Tanaka, Hiroshi; Mitsunobu, Fumihiro*; Yamaoka, Kiyonori*
Journal of Radiation Research, 52(6), p.775 - 781, 2011/11
Times Cited Count:26 Percentile:67.24(Biology)We examined dose-dependent or dose rate-dependent changes of superoxide dismutase (SOD) activity using a new large-scale facility for exposing small animals to radon. Mice were exposed to radon at a concentration of 250, 500, 1000, 2000, or 4000 Bq/m for 0.5, 1, 2, 4, or 8 days. When mice were exposed to radon at 2000 day Bq/m
for 0.5, 1, 2, 4, or 8 days. When mice were exposed to radon at 2000 day Bq/m , activation of SOD activities in plasma, liver, pancreas, heart, thymus, and kidney showed dose-rate effects. Our results also suggested that continuous exposure to radon increased SOD activity, but SOD activity transiently returned to normal levels at around 2 days. Moreover, we classified the organs into four groups ((1) plasma, brain, lung (2) heart, liver, pancreas, small intestine (3) kidney, thymus (4) stomach) based on changes in SOD activity. Thymus had the highest responsiveness and stomach had lowest. These data provide useful baseline measurements for future studies on radon effects.
, activation of SOD activities in plasma, liver, pancreas, heart, thymus, and kidney showed dose-rate effects. Our results also suggested that continuous exposure to radon increased SOD activity, but SOD activity transiently returned to normal levels at around 2 days. Moreover, we classified the organs into four groups ((1) plasma, brain, lung (2) heart, liver, pancreas, small intestine (3) kidney, thymus (4) stomach) based on changes in SOD activity. Thymus had the highest responsiveness and stomach had lowest. These data provide useful baseline measurements for future studies on radon effects.
Ishimori, Yuu; Mitsunobu, Fumihiro*; Yamaoka, Kiyonori*; Tanaka, Hiroshi; Kataoka, Takahiro*; Sakoda, Akihiro*
Radiation Protection Dosimetry, 146(1-3), p.31 - 33, 2011/07
Times Cited Count:12 Percentile:66.82(Environmental Sciences)A radon test facility for small animals was developed in order to increase the statistical validity of differences of the biological response in various radon environments. This paper illustrates the performances of that facility, the first large-scale facility of its kind in Japan. The facility has a capability to conduct approximately 150 mouse-scale tests at the same time. The apparatus for exposing small animals to radon has six animal chamber groups with five independent cages each. Different radon concentrations in each animal chamber group are available. Because the first target of this study is to examine the in vivo behavior of radon and its effects, the major functions to control radon and to eliminate thoron were examined experimentally. Additionally, radon progeny concentrations and their particle size distributions in the cages were also examined experimentally to be considered in future projects.
Ishimori, Yuu; Mitsunobu, Fumihiro*; Yamaoka, Kiyonori*; Tanaka, Hiroshi; Kataoka, Takahiro*; Sakoda, Akihiro*
Hoken Butsuri, 45(1), p.65 - 71, 2010/03
Japan Atomic Energy Agency (JAEA) and Okayama University have carried out the experimental animal study and its related studies since 2007 in order to examine the physical effect of radon in detail. Thus, a radon test facility for small animals was developed in order to increase the statistical certainty of our animal tests. This paper illustrates the performance of the facility, the first large-scale facility in Japan. The facility has a potential of about 150 mouse-scale test at the same time. Different concentration at each animal chamber group is available. Controlling radon and avoiding thoron were theoretically and experimentally shown as the fundamental performance of the facility. The relative standard deviation of the radon concentration at the highest concentration group was about 5%, although the lower concentration groups seemed to be affected by variation of background.