Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
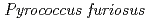 by BIX-3, a single-crystal diffractometer for biomacromolecules
by BIX-3, a single-crystal diffractometer for biomacromoleculesKurihara, Kazuo; Tanaka, Ichiro*; Chatake, Toshiyuki*; Adams, M. W. W.*; Jenney, F. E. Jr.*; Moiseeva, N.*; Bau, R.*; Niimura, Nobuo
Proceedings of the National Academy of Sciences of the United States of America, 101(31), p.11215 - 11220, 2004/08
Times Cited Count:48 Percentile:61.13(Multidisciplinary Sciences)The structure of a rubredoxin (Rd) from 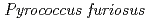 , an organism that grows optimally at 100
, an organism that grows optimally at 100  C, was determined using the neutron single-crystal diffractometer for biological macromolecules (BIX-3) at the JRR-3 reactor of JAERI. Data were collected at room temperature up to a resolution of 1.5
C, was determined using the neutron single-crystal diffractometer for biological macromolecules (BIX-3) at the JRR-3 reactor of JAERI. Data were collected at room temperature up to a resolution of 1.5  , and the completeness of the data set was 81.9 %. The model contains 306 H atoms and 50 D atoms. A total of 37 hydration water molecules were identified. The model has been refined to final agreement factors of
, and the completeness of the data set was 81.9 %. The model contains 306 H atoms and 50 D atoms. A total of 37 hydration water molecules were identified. The model has been refined to final agreement factors of  = 18.6 % and
= 18.6 % and 
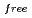 = 21.7 %. Several orientations of the O-D bonds of side chains, whose assignments from X-ray data were previously ambiguous, were clearly visible in the neutron structure. While most backbone N-H bonds had undergone some degree of H/D exchange throughout the molecule, five H atom positions still had distinctly negative (H) peaks. The neutron Fourier maps clearly showed the details of an extensive set of H bonds involving the ND
= 21.7 %. Several orientations of the O-D bonds of side chains, whose assignments from X-ray data were previously ambiguous, were clearly visible in the neutron structure. While most backbone N-H bonds had undergone some degree of H/D exchange throughout the molecule, five H atom positions still had distinctly negative (H) peaks. The neutron Fourier maps clearly showed the details of an extensive set of H bonds involving the ND
 terminus that may contribute to the unusual thermostability of this molecule.
terminus that may contribute to the unusual thermostability of this molecule.
Kurihara, Kazuo; Tanaka, Ichiro; Adams, M. W. W.*; Jenney, F. E. Jr.*; Moiseeva, N.*; Bau, R.*; Niimura, Nobuo
Journal of the Physical Society of Japan, Vol.70, Supplement A, p.400 - 402, 2001/05
With the new single-crystal diffractometer BIX-3 at the JRR-3M reactor of JAERI, a single-crystal neutron diffraction analysis of the structure of the small protein rubredoxin from the hyperthermophile Pyrococcus furiosus is currently under way. Data were collected at room temperature up to a resolution of 1.5  intervals in
intervals in 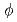 and exposure times ranging from 60 to 77 minutes per frame. The completeness factor of the 1.5-
and exposure times ranging from 60 to 77 minutes per frame. The completeness factor of the 1.5- . Included in the refinement are 301 hydrogen atoms and 40 deuterium atoms, and 29 water molecules were also identified. In the present model, the current value for R and R
. Included in the refinement are 301 hydrogen atoms and 40 deuterium atoms, and 29 water molecules were also identified. In the present model, the current value for R and R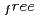 are 24.0
are 24.0 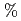 and 26.3
and 26.3 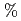 , respectively.
, respectively.