Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 Be activity concentrations in Dazaifu (western Japan)
Be activity concentrations in Dazaifu (western Japan)Narazaki, Yukinori*; Sakoda, Akihiro; Akata, Naofumi*; Ito, Hisanori*; Momoshima, Noriyuki*
Journal of Environmental Radioactivity, 284, p.107612_1 - 107612_7, 2025/04
Times Cited Count:0 Percentile:0.00(Environmental Sciences)Atmospheric  Be activity concentration was continuously measured in Dazaifu, western Japan, from 1999 to 2020, and its variation was analyzed. Daily
Be activity concentration was continuously measured in Dazaifu, western Japan, from 1999 to 2020, and its variation was analyzed. Daily  Be data analysis, encompassing an analysis for 22 years, revealed a concentration range of not detected - 18 mBq/m
Be data analysis, encompassing an analysis for 22 years, revealed a concentration range of not detected - 18 mBq/m , characterized by substantial monthly variation and smoothed annual variation. An average daily
, characterized by substantial monthly variation and smoothed annual variation. An average daily  Be activity concentration of 5.0
Be activity concentration of 5.0  2.6 mBq/m
2.6 mBq/m was considered to be representative at the ground-surface-level in 30-40
was considered to be representative at the ground-surface-level in 30-40 N. Results from a two-way Analysis of variance (ANOVA) indicated statistical significance in monthly and annual
N. Results from a two-way Analysis of variance (ANOVA) indicated statistical significance in monthly and annual  Be variabilities. The monthly variability of
Be variabilities. The monthly variability of  Be activity concentration was approximately four times greater than the annual variability. Frequency analysis revealed that the monthly variability comprised major 12-month and minor 6-month periodicities. The substantial decrease in
Be activity concentration was approximately four times greater than the annual variability. Frequency analysis revealed that the monthly variability comprised major 12-month and minor 6-month periodicities. The substantial decrease in  Be activity concentration during summer, a primary driver of monthly variation, was also observed at other locations in Japan, attributed to a stable high-pressure system in the Pacific Ocean that stalled over Japan's southern seas, followed by the inflow of air masses containing low
Be activity concentration during summer, a primary driver of monthly variation, was also observed at other locations in Japan, attributed to a stable high-pressure system in the Pacific Ocean that stalled over Japan's southern seas, followed by the inflow of air masses containing low  Be activity concentrations. The annual variation was primarily influenced by the 11-year solar activity cycle, which affects the intensity of cosmic rays that produce
Be activity concentrations. The annual variation was primarily influenced by the 11-year solar activity cycle, which affects the intensity of cosmic rays that produce  Be.
Be.
 Be activity concentrations with respect to precipitation
Be activity concentrations with respect to precipitationNarazaki, Yukinori*; Sakoda, Akihiro; Akata, Naofumi*; Ito, Hisanori*; Momoshima, Noriyuki*
Journal of Environmental Radioactivity, 277, p.107432_1 - 107432_7, 2024/07
Beryllium-7 activity concentrations in the atmosphere and precipitation were continuously measured every day between April 2011 and December 2015 in Dazaifu, western Japan. The measured data were quantitatively analyzed to determine the precipitation-induced variation in  Be activity concentrations. The average concentrations on nonprecipitation and precipitation days were 5.5 and 3.8 mBq/m
Be activity concentrations. The average concentrations on nonprecipitation and precipitation days were 5.5 and 3.8 mBq/m , respectively. This difference of 31% (1.7 mBq/m
, respectively. This difference of 31% (1.7 mBq/m ) on average, was attributable to the washout effect, which was more significant in the summer. Regarding the association between
) on average, was attributable to the washout effect, which was more significant in the summer. Regarding the association between  Be activity concentration and precipitation, the concentration remained at a similar level for the small precipitation amount of
Be activity concentration and precipitation, the concentration remained at a similar level for the small precipitation amount of  5.0 mm/day and showed a decreasing trend (but was insignificant) for the precipitation of 5.0-10.0 mm/day. A significant decrease in the concentration was observed for
5.0 mm/day and showed a decreasing trend (but was insignificant) for the precipitation of 5.0-10.0 mm/day. A significant decrease in the concentration was observed for 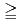 10 mm/day. Furthermore, when precipitation occurred on two successive days, the
10 mm/day. Furthermore, when precipitation occurred on two successive days, the  Be activity concentrations on the second day significantly decreased regardless of precipitation.
Be activity concentrations on the second day significantly decreased regardless of precipitation.
 Be activity concentrations in the atmosphere
Be activity concentrations in the atmosphereNarazaki, Yukinori*; Sakoda, Akihiro; Akata, Naofumi*; Ito, Hisanori*; Momoshima, Noriyuki*
International Journal of Environmental Research and Public Health, 19(16), p.10128_1 - 10128_9, 2022/08
Times Cited Count:4 Percentile:45.94(Environmental Sciences)In March 2013, increased  Be activity concentrations in the atmosphere were observed for successive days in Dazaifu, western Japan. The daily
Be activity concentrations in the atmosphere were observed for successive days in Dazaifu, western Japan. The daily  Be activity concentration averages ranged from 0.93 to 14 mBq/m
Be activity concentration averages ranged from 0.93 to 14 mBq/m , with a monthly average of 8.3 mBq/m
, with a monthly average of 8.3 mBq/m . This average was the highest among the monthly averages observed between 1999 and 2015 and higher than the monthly average over this period (4.7 mBq/m
. This average was the highest among the monthly averages observed between 1999 and 2015 and higher than the monthly average over this period (4.7 mBq/m ) plus twice the standard deviation (1.7
) plus twice the standard deviation (1.7  2 mBq/m
2 mBq/m = 8.1 mBq/m
= 8.1 mBq/m ). Also, this exceeded the monthly average (6.0 mBq/m
). Also, this exceeded the monthly average (6.0 mBq/m ) only for March 1999-2015, excluding 2013, where the cosmic-ray intensity, a component producing
) only for March 1999-2015, excluding 2013, where the cosmic-ray intensity, a component producing  Be, decreased. Based on the backward trajectory analysis results, the inflow of air from the stratosphere and upper troposphere at high latitudes that frequently occurred in March 2013 was considered the reason for the
Be, decreased. Based on the backward trajectory analysis results, the inflow of air from the stratosphere and upper troposphere at high latitudes that frequently occurred in March 2013 was considered the reason for the  Be activity concentration increase.
Be activity concentration increase.
 Be; Particle size distribution and chemical composition of
Be; Particle size distribution and chemical composition of  Be-carrying aerosols in the atmosphere in Japan
Be-carrying aerosols in the atmosphere in JapanNarazaki, Yukinori*; Sakoda, Akihiro; Takahashi, Shunta*; Momoshima, Noriyuki*
Journal of Environmental Radioactivity, 237, p.106690_1 - 106690_7, 2021/10
Times Cited Count:7 Percentile:31.98(Environmental Sciences)The particle size distributions of airborne aerosols with  Be were measured using cascade impactors at Dazaifu, a city in western Japan, in 2018 to observe their seasonal variation.
Be were measured using cascade impactors at Dazaifu, a city in western Japan, in 2018 to observe their seasonal variation.  Be was found to be attached to aerosols with a particle size of less than 2.1
Be was found to be attached to aerosols with a particle size of less than 2.1  m; in general, particles sized 0.43-0.65
m; in general, particles sized 0.43-0.65  m had the highest
m had the highest  Be activity concentrations. The activity median aerodynamic diameter (AMAD) of
Be activity concentrations. The activity median aerodynamic diameter (AMAD) of  Be was in the range of 0.39-0.52
Be was in the range of 0.39-0.52  m, which is the size range of particles that can reach human alveoli, and had an annual mean of 0.43
m, which is the size range of particles that can reach human alveoli, and had an annual mean of 0.43 0.035
0.035  m. The activity concentrations of
m. The activity concentrations of  Be were significantly lower in summer, which affected its activity concentrations in the particle size distributions of
Be were significantly lower in summer, which affected its activity concentrations in the particle size distributions of  Be. The particle size distribution of
Be. The particle size distribution of  Be-carrying aerosols was also affected by that of the aerosol particles in the atmosphere. Finally, findings suggest that
Be-carrying aerosols was also affected by that of the aerosol particles in the atmosphere. Finally, findings suggest that  Be was mainly attached to sulfate aerosols (particularly ammonium sulfate aerosols).
Be was mainly attached to sulfate aerosols (particularly ammonium sulfate aerosols).
Nakasone, Shunya*; Yokoyama, Sumi*; Takahashi, Tomoyuki*; Ota, Masakazu; Kakiuchi, Hideki*; Sugihara, Shinji*; Hirao, Shigekazu*; Momoshima, Noriyuki*; Tamari, Toshiya*; Shima, Nagayoshi*; et al.
Plasma and Fusion Research (Internet), 16, p.2405035_1 - 2405035_5, 2021/02
Removal of impurities such as organic and other types of dissolved matters from environmental water samples is required for precise analysis of tritium with a liquid scintillation counting method. In general, a distillation method is a conventional one for tritium analysis in environmental water samples, but is a time-consuming process that takes 24 hours for removal of impurities. We have proposed a rapid pretreatment method for tritium analysis, that uses ion exchange resins. In this study, we performed batch experiments, to evaluate the effectiveness of the ion exchange resins on the tritium measurement. The results obtained demonstrated that removal of impurities in the sample water by ion exchange resins can be achieved during a short period of time (i.e., in 5 min).
Nakasone, Shunya*; Yokoyama, Sumi*; Takahashi, Tomoyuki*; Ota, Masakazu; Kakiuchi, Hideki*; Sugihara, Shinji*; Hirao, Shigekazu*; Momoshima, Noriyuki*; Tamari, Toshiya*; Shima, Nagayoshi*; et al.
Plasma and Fusion Research (Internet), 15, p.2405027_1 - 2405027_3, 2020/05
A quick preprocessing system for tritium analysis of environmental samples is important to judge environmental influence of tritium releases due to accident or tritium-handling facilities. Analysis of tritium in water samples with liquid scintillation counting method requires removal of impurities such as organic matter and ion species from water samples. Generally, a distillation method is adopted as a pretreatment of analysis for tritium; however, the distillation method is a time-consuming process. The aim of this study is to evaluate a rapid pretreatment method for tritium analysis with ion exchange resin. From batch and column experiments that used inland water and ion exchange resin, we confirmed removals of impurities of the water sample and that the removal of impurities was possible for a short time (by 5 minutes).
Yokoyama, Sumi*; Takahashi, Tomoyuki*; Ota, Masakazu; Kakiuchi, Hideki*; Sugihara, Shinji*; Hirao, Shigekazu*; Momoshima, Noriyuki*; Tamari, Toshiya*; Shima, Nagayoshi*; Atarashi-Andoh, Mariko; et al.
Plasma and Fusion Research (Internet), 14(Sp.2), p.3405099_1 - 3405099_4, 2019/06
The Large Helical Device of the National Institute for Fusion Science started D-D experiments in 2017. To ensure the safety of the facility, it is important to develop evaluation methods for environmental tritium transfer. Tritiated water (HTO) in atmosphere and soil is transferred to plants, and organically bound tritium (OBT) is formed by photosynthesis. Prediction of OBT formation is important, because OBT accumulates in plants and causes dose through ingestion. The objective of this study is to estimate environmental tritium transfer using a simple compartment model and practical parameters. We proposed a simple compartment model consisting of air-soil-plant components, and tried to validate the model by comparison with a sophisticated model, SOLVEG. In this study, we plan to add wet deposition to the model and obtain parameters from measurements of soil permeability and tritium concentrations in air, soil and plants. We also establish rapid pretreatment methods for OBT analysis.
Sugihara, Shinji*; Tanaka, Masahiro*; Tamari, Toshiya*; Shimada, Jun*; Takahashi, Tomoyuki*; Momoshima, Noriyuki*; Fukutani, Satoshi*; Atarashi-Andoh, Mariko; Sakuma, Yoichi*; Yokoyama, Sumi*; et al.
Fusion Science and Technology, 60(4), p.1300 - 1303, 2011/11
Times Cited Count:2 Percentile:17.59(Nuclear Science & Technology)The purpose of this study is to develop the technique to evaluate the environmental tritium behavior of the nuclear facility origin. Tritium concentrations of river water, precipitation and ground water around the NIFS site were determined by low background liquid scintillation measurement system combined with the electrolysis using solid polymer electrolyte. The electric conductivity and flow rate of the river and isotopic ratio of oxygen and hydrogen of water samples were also measured. The tritium concentrations in precipitation showed the seasonal variation and the range were 0.09-0.78 Bq/L. The tritium concentrations of river water and ground water were almost constant, 0.34 and 0.24 Bq/L respectively. The simple dynamic model for the site around the NIFS facilities was developed using measured data, and the behavior of tritium was simulated.
Momoshima, Noriyuki*; Hayashi, Takumi
Purazuma, Kaku Yugo Gakkai-Shi, 85(1), p.36 - 40, 2009/01
no abstracts in English
Amano, Hikaru; Yamamichi, Miwako*; Baba, Masami*; Momoshima, Noriyuki*; Sugihara, Shinji*; Ueda, Yusuke*; Nakamura, Yasuhiro*
JAEA-Conf 2008-003, p.84 - 87, 2008/04
no abstracts in English
Shima, Shigeki*; Gasa, Shinichi*; Amano, Hikaru; Nagao, Seiya*; Yamamoto, Masayoshi*; Momoshima, Noriyuki*; Furukawa, Masahide*; Kimura, Hideki*; Kawamura, Hisao*
JAEA-Conf 2008-003, p.28 - 31, 2008/04
Concentrations of  I in surface seawater around Japan were approximately 2
I in surface seawater around Japan were approximately 2 10
10 atoms/L in literatures. However, the atomic ratio of iodine to cesium was ten times as high as that of the global fallout. The origin of
atoms/L in literatures. However, the atomic ratio of iodine to cesium was ten times as high as that of the global fallout. The origin of  I in the water columns seems to be difficult to be explained by only the global fallout. Discharge from European plants was one of the possible origins of iodine from the standpoint of air mass trajectory analysis. Concentration of
I in the water columns seems to be difficult to be explained by only the global fallout. Discharge from European plants was one of the possible origins of iodine from the standpoint of air mass trajectory analysis. Concentration of  I in rain water was 10 times higher than that in surface seawater. Anthropogenic inorganic iodine in surface seawater predominantly dissolves as an iodide ion (I
I in rain water was 10 times higher than that in surface seawater. Anthropogenic inorganic iodine in surface seawater predominantly dissolves as an iodide ion (I ).
).
Kakiuchi, Hideki*; Tanaka, Masahiro*; Fukutani, Satoshi*; Sugihara, Shinji*; Hirao, Shigekazu*; Momoshima, Noriyuki*; Tamari, Toshiya*; Shima, Nagayoshi*; Atarashi-Andoh, Mariko; Furukawa, Masahide*; et al.
no journal, ,
no abstracts in English
Kokubu, Yoko; Momoshima, Noriyuki*; Hirose, Katsumi*; Tagami, Keiko*; Takamiya, Koichi*
no journal, ,
Just after an accident at the Fukushima Daiichi Nuclear Power Plant (FDNPP), data of radionuclides released from the FDNPP were measured for environmental samples by some researchers at individual research institute and university. However a comprehensive database including these data is not constructed now. Working group of the Japan Society of Nuclear and Radiochemical Sciences decided to construct a comprehensive database of data of the radionuclides in environmental samples. This database contains data such as sampling location, sampling method, sampling times, type of measurement system, concentrations of the radionuclides and others in the publications/reports and unpublished issues. Researchers input the data in a dedicated Excel sheet. The working group verifies and registers the data in the database, and then summarizes them in a scientific report.
Yokoyama, Sumi*; Takahashi, Tomoyuki*; Ota, Masakazu; Kakiuchi, Hideki*; Sugihara, Shinji*; Hirao, Shigekazu*; Momoshima, Noriyuki*; Tamari, Toshiya*; Shima, Nagayoshi*; Atarashi-Andoh, Mariko; et al.
no journal, ,
To ensure safety of fusion facilities, it is important to develop evaluation methods for tritium transfer in the environment. For estimation of tritium transfer in the terrestrial environment, we had developed a simple compartment model using the Migration Of GRound Additions (MOGRA) code. The model was composed by an air-soil-plant system. The target source terms were HT and HTO in the air. In addition, wet deposition was modeled by input of HTO to the system by rainfall. Tritium in the plant was divided into free water tritium (FWT) and organically bound tritium (OBT). The tritium concentration in the environmental medium was trial calculated for chronic and accidental HTO releases to the atmosphere, as preliminary calculation run of the model.