Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Osaka, Masahiko; Miwa, Shuhei; Tanaka, Kosuke; Sato, Isamu; Hirosawa, Takashi; Obayashi, Hiroshi; Mondo, Kenji; Akutsu, Yoko; Ishi, Yohei; Koyama, Shinichi; et al.
WIT Transactions on Ecology and the Environment, Vol.105, p.357 - 366, 2007/06
no abstracts in English
Sato, Isamu; Seki, Takayuki*; Ishi, Yohei; Mondo, Kenji; Yoshimochi, Hiroshi; Tanaka, Kenya
JAEA-Research 2007-013, 63 Pages, 2007/03
In air atmosphere, the weight of Am-MOX fuel relatively rapidly increased, which change rate strongly depended on the initial O/M ratio. The lower initial O/M ratio is, the higher the rate is. However, for the other MOX fuel containing little Am, the equivalent behavior have been observed, which indicated that not only Am but also the property of law material powder affected the behavior. The X-ray diffraction pattern change as time goes by was observed, which was a evidence that the O/M ratio change might arise from crystallographic one. The rate of O/M ratio change is a function of water vapor pressure in the atmosphere. If the water vapor pressure would is set to be quite low (ex.  1ppm), the O/M ratio change could effectively been avoided. On a model basis of Am(III) and U(V) existence, we could explain the O/M ratio dependence of the lattice parameter of Am-MOX fuel near O/M ratio, 2.00 better.
1ppm), the O/M ratio change could effectively been avoided. On a model basis of Am(III) and U(V) existence, we could explain the O/M ratio dependence of the lattice parameter of Am-MOX fuel near O/M ratio, 2.00 better.
 by an in-cell remote process
by an in-cell remote processYoshimochi, Hiroshi; Ishi, Yohei; Seki, Takayuki*; Mondo, Kenji; Sekine, Shinichi*; Koyama, Shinichi
Saikuru Kiko Giho, (28), p.9 - 20, 2005/09
An in-cell remote fabrication technique has been developed for MOX fuel pellets containing 3 and 5% americium (Am-MOX) at the Alpha-gamma Facility (AGF) in O-arai Engineering Center. A series of fuel pellet and the pin fabrication apparatuses were systematically installed in hot cell to make fabrication flow easier. After cold run and some modifications, they were remotely controlled from a panel in the operation room outside the hot cell as much as possible. From a preliminary UO2 pellet test and consequently plutonium pellet fabrication run, actual range of ball milling time, pressing and sintering condition were focused for Am-MOX pellet fabrication. As the next step, moisturized atmosphere was found out to remove the heterogeneity structure of 5% Am-MOX pellet. Finally, we established an optimized fabrication condition of 5% Am-MOX pellet sintered at 1700 C for 3h in an atmosphere of 5% H2-95% Ar with total moisture of 2000 ppm. Moreover it is important that the atmosphere has to be changed to dry gas at 800
C for 3h in an atmosphere of 5% H2-95% Ar with total moisture of 2000 ppm. Moreover it is important that the atmosphere has to be changed to dry gas at 800 C during cool down.
C during cool down.

Osaka, Masahiko; Miwa, Shuhei; Mondo, Kenji; Ozaki, Yoko; Ishi, Yohei; Yoshimochi, Hiroshi; Seki, Takayuki*; Sekine, Shinichi*; Ishida, Takashi*; Tanaka, Kenya
JNC TN9400 2005-002, 40 Pages, 2005/03
An experimental investigation for the phase relation of (U,Pu,Am)O was performed with XRD, ceramography and DTA. Although lattice parameter tended to increase with increases of Am content and O/M ratio, its slope differed from that of (U,Pu)O
was performed with XRD, ceramography and DTA. Although lattice parameter tended to increase with increases of Am content and O/M ratio, its slope differed from that of (U,Pu)O . A drastic structural change was observed around O/M=1.98. Besides, many DTA peaks, which could never be seen in the case of (U,Pu)O
. A drastic structural change was observed around O/M=1.98. Besides, many DTA peaks, which could never be seen in the case of (U,Pu)O , were observed above O/M=1.98.These results were interpreted with a hypothesis that all Am were trivalent and equivalent amount of U became pentavalent. The dependence of lattice parameter on Am content could be expressed well by using a model with ionic radii of each element. Also the structural change around O/M=1.98 could be explained as caused by valence states of each element. It was concluded from these interpretation that all Am in (U,Pu,Am)O
, were observed above O/M=1.98.These results were interpreted with a hypothesis that all Am were trivalent and equivalent amount of U became pentavalent. The dependence of lattice parameter on Am content could be expressed well by using a model with ionic radii of each element. Also the structural change around O/M=1.98 could be explained as caused by valence states of each element. It was concluded from these interpretation that all Am in (U,Pu,Am)O were likely to exist as trivalent state.
were likely to exist as trivalent state.
 by an in-cell remote process
by an in-cell remote processYoshimochi, Hiroshi; Nemoto, Masanao; Mondo, Kenji; Koyama, Shinichi; Namekawa, Takashi
Journal of Nuclear Science and Technology, 41(8), p.850 - 856, 2004/08
Focusing on the cover layer materials (as the Radon Barrier Materials), which could have the effect to restrain the radon from scattering into the air and the effect of the radiation shielding, we produced the radon barrier materials with crude bentonite on an experimental basis, using the rotary type comprehensive unit for grinding and mixing, through which we carried out the evaluation of the characteristics thereof.
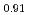 Am
Am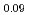 )O
)O
Osaka, Masahiko; Mondo, Kenji; Ishi, Yohei; Tanaka, Kenya; Seki, Takayuki*; Kurosaki, Ken*; Uno, Masayoshi*; Yamanaka, Shinsuke*
no journal, ,
Oxygen potential of (Pu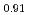 Am
Am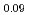 )O
)O were measured by thermogravimetry using H
were measured by thermogravimetry using H O/H
O/H gas equiribrium, as a fundamental part of investigation on phase relation for low-decontaminated fuel.
gas equiribrium, as a fundamental part of investigation on phase relation for low-decontaminated fuel.
Sato, Isamu; Ishi, Yohei; Mondo, Kenji; Yoshimochi, Hiroshi; Tanaka, Kenya; Seki, Takayuki*
no journal, ,
Wieght changes of MOX fuels containing Am were measured during stored in a cell box. We observed that the O/M ratios of these fuels containing Am changed relatively rapidly.
Mondo, Kenji; Ishi, Yohei; Sato, Isamu; Yoshimochi, Hiroshi; Tanaka, Kenya; Seki, Takayuki*
no journal, ,
no abstracts in English
Sato, Isamu; Ishi, Yohei; Mondo, Kenji; Tanaka, Kenya; Seki, Takayuki*; Ishida, Takashi*
no journal, ,
A dependence of O/M ratio change behavior on atmosphere was evaluated, then these results indicated that the change rate of the O/M ratio depended on the concentration of water.
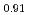 Am
Am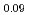 )O
)O
Miwa, Shuhei; Osaka, Masahiko; Mondo, Kenji; Ishi, Yohei; Yoshimochi, Hiroshi; Tanaka, Kenya; Seki, Takayuki*; Sekine, Shinichi*; Kurosaki, Ken*; Uno, Masayoshi*; et al.
no journal, ,
An experimental investigation on phase relation of (Pu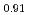 Am
Am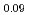 )O
)O was carried out by X-ray diffraction (XRD) analysis, ceramography and Differential Thermal Analysis (DTA) for (Pu
was carried out by X-ray diffraction (XRD) analysis, ceramography and Differential Thermal Analysis (DTA) for (Pu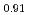 Am
Am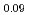 )O
)O having different O/M ratios from 1.90 to 2.00.
having different O/M ratios from 1.90 to 2.00.