Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Sugita, Tsuyoshi; Kobayashi, Kentaro*; Yamazaki, Taiki*; Isaka, Mayu*; Itabashi, Hideyuki*; Mori, Masanobu*
Journal of Photochemistry and Photobiology A; Chemistry, 400, p.112662_1 - 112662_8, 2020/09
Times Cited Count:1 Percentile:2.52(Chemistry, Physical)In this study, we developed an in-line photocatalytic performance evaluation system in which a flow reactor was connected to the ion chromatography to accurately evaluate the performance of the photocatalyst. This system was used to evaluate the photocatalyst supported by the two-layer support method on the substrate, such as glass beads. The performance of the photocatalyst was evaluated using dimethyl sulfoxide (DMSO), and it was possible to monitor the decomposition of DMSO by UV and the formation of by-products, such as methane sulfonate (MSO) and sulfate (SA). This system can be expected to be useful not only for evaluating the decomposition performance of an object using a photocatalyst but also for evaluating the byproducts.
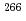 Bh in the
Bh in the  Cm(
Cm(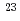 Na,5
Na,5 )
)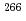 Bh reaction and its decay properties
Bh reaction and its decay propertiesHaba, Hiromitsu*; Fan, F.*; Kaji, Daiya*; Kasamatsu, Yoshitaka*; Kikunaga, Hidetoshi*; Komori, Yukiko*; Kondo, Narumi*; Kudo, Hisaaki*; Morimoto, Koji*; Morita, Kosuke*; et al.
Physical Review C, 102(2), p.024625_1 - 024625_12, 2020/08
Times Cited Count:6 Percentile:59.56(Physics, Nuclear)Tanaka, Taiki*; Morita, Kosuke*; Morimoto, Koji*; Kaji, Daiya*; Haba, Hiromitsu*; Boll, R. A.*; Brewer, N. T.*; Van Cleve, S.*; Dean, D. J.*; Ishizawa, Satoshi*; et al.
Physical Review Letters, 124(5), p.052502_1 - 052502_6, 2020/02
Times Cited Count:19 Percentile:81.08(Physics, Multidisciplinary) -coated wire mesh prepared via a double-layer coating method
-coated wire mesh prepared via a double-layer coating methodMori, Masanobu*; Sugita, Tsuyoshi; Fujii, Kengo*; Yamazaki, Taiki*; Isaka, Mayu*; Kobayashi, Kentaro*; Iwamoto, Shinji*; Itabashi, Hideyuki*
Analytical Sciences, 34(12), p.1449 - 1453, 2018/12
Times Cited Count:2 Percentile:7.07(Chemistry, Analytical)The photocatalyst coating stainless-steel wire mesh (TiO -WM) was prepared by double-layer coating method. The TiO
-WM) was prepared by double-layer coating method. The TiO -WM was evaluated using flow analytical system, which included the reactor and conductimetric detector (FAS-CD). The DMSO decomposition test through the FAS-CD reveal that photocatalytst was stable coating on the stainless-steel wire mesh.
-WM was evaluated using flow analytical system, which included the reactor and conductimetric detector (FAS-CD). The DMSO decomposition test through the FAS-CD reveal that photocatalytst was stable coating on the stainless-steel wire mesh.
 as anionic adsorptive photocatalyst
as anionic adsorptive photocatalystSugita, Tsuyoshi; Kobayashi, Kenichi*; Kobayashi, Kentaro*; Yamazaki, Taiki*; Fujii, Kengo*; Itabashi, Hideyuki*; Mori, Masanobu*
Journal of Photochemistry and Photobiology A; Chemistry, 356, p.71 - 80, 2018/04
Times Cited Count:5 Percentile:13.28(Chemistry, Physical)Photocatalysts shows high redox property only by light irradiation. However, the reaction performance is lowered in aqueous phase due to the low contact efficiency between catalyst and targets. In this study, to enhance aqueous adsorption and photodecomposition of anionic organic target, we developed an amino functional-based spacer (3-[2-(2- aminoethylamino)ethylamino]propyl-trimethoxysilane, DETA), and used it to modify TiO . The modified catalyst with positively charged amino groups could enhance the adsorption and photodecomposition of anionic organic targets.
. The modified catalyst with positively charged amino groups could enhance the adsorption and photodecomposition of anionic organic targets.
Tanaka, Taiki*; Narikiyo, Yoshihiro*; Morita, Kosuke*; Fujita, Kunihiro*; Kaji, Daiya*; Morimoto, Koji*; Yamaki, Sayaka*; Wakabayashi, Yasuo*; Tanaka, Kengo*; Takeyama, Mirei*; et al.
Journal of the Physical Society of Japan, 87(1), p.014201_1 - 014201_9, 2018/01
Times Cited Count:18 Percentile:74.47(Physics, Multidisciplinary)Excitation functions of quasielastic scattering cross sections for the  Ca +
Ca +  Pb,
Pb,  Ti +
Ti +  Pb, and
Pb, and  Ca +
Ca +  Cm reactions were successfully measured by using the gas-filled recoil-ion separator GARIS. Fusion barrier distributions were extracted from these data, and compared with the coupled-channels calculations. It was found that the peak energies of the barrier distributions for the
Cm reactions were successfully measured by using the gas-filled recoil-ion separator GARIS. Fusion barrier distributions were extracted from these data, and compared with the coupled-channels calculations. It was found that the peak energies of the barrier distributions for the  Ca +
Ca +  Pb and
Pb and  Ti +
Ti +  Pb systems coincide with those of the 2n evaporation channel cross sections for the systems, while that of the
Pb systems coincide with those of the 2n evaporation channel cross sections for the systems, while that of the  Ca +
Ca +  Cm is located slightly below the 4n evaporation ones. This results provide us helpful information to predict the optimum beam energy to synthesize superheavy nuclei.
Cm is located slightly below the 4n evaporation ones. This results provide us helpful information to predict the optimum beam energy to synthesize superheavy nuclei.
 Ca +
Ca +  Cm
Cm 
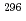 Lv
Lv at RIKEN-GARIS
at RIKEN-GARISKaji, Daiya*; Morita, Kosuke*; Morimoto, Koji*; Haba, Hiromitsu*; Asai, Masato; Fujita, Kunihiro*; Gan, Z.*; Geissel, H.*; Hasebe, Hiroo*; Hofmann, S.*; et al.
Journal of the Physical Society of Japan, 86(3), p.034201_1 - 034201_7, 2017/03
Times Cited Count:28 Percentile:81.43(Physics, Multidisciplinary)The fusion reaction of  Ca +
Ca +  Cm
Cm 
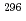 Lv
Lv was studied using the gas-filled recoil-ion separator GARIS at RIKEN. A total of seven
was studied using the gas-filled recoil-ion separator GARIS at RIKEN. A total of seven  and spontaneous-fission decay chains were observed, which would originate from the reaction products of the element 116,
and spontaneous-fission decay chains were observed, which would originate from the reaction products of the element 116, 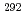 Lv and
Lv and 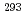 Lv. Decay properties observed in the chains are in good agreement with the previously published ones. However, one of the chains showed a discrepancy, indicating the new spontaneous-fission branch in
Lv. Decay properties observed in the chains are in good agreement with the previously published ones. However, one of the chains showed a discrepancy, indicating the new spontaneous-fission branch in 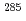 Cn or the production of the new isotope
Cn or the production of the new isotope 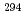 Lv.
Lv.
 -orbital symmetry of the ground state in Yb compounds by linear dichroism in core-level photoemission
-orbital symmetry of the ground state in Yb compounds by linear dichroism in core-level photoemissionMori, Takeo*; Kitayama, Satoshi*; Kanai, Yuina*; Naimen, Sho*; Fujiwara, Hidenori*; Higashiya, Atsushi*; Tamasaku, Kenji*; Tanaka, Arata*; Terashima, Kensei*; Imada, Shin*; et al.
Journal of the Physical Society of Japan, 83(12), p.123702_1 - 123702_5, 2014/12
Times Cited Count:16 Percentile:68.53(Physics, Multidisciplinary)We show that the strongly correlated 4 -orbital symmetry of the ground state is revealed by linear dichroism in core-level photoemission spectra, as we have discovered for YbRh
-orbital symmetry of the ground state is revealed by linear dichroism in core-level photoemission spectra, as we have discovered for YbRh Si
Si and YbCu
and YbCu Si
Si . Theoretical analysis shows us that the linear dichroism reflects the anisotropic charge distributions resulting from a crystalline electric field. We have successfully determined the ground-state 4
. Theoretical analysis shows us that the linear dichroism reflects the anisotropic charge distributions resulting from a crystalline electric field. We have successfully determined the ground-state 4 symmetry for both compounds from the polarization-dependent angle resolved core-level spectra at a temperature well below the first excitation energy. The excited-state symmetry is also probed by temperature dependence of the linear dichroism where the high measurement temperatures are on the order of the crystal-field-splitting energies.
symmetry for both compounds from the polarization-dependent angle resolved core-level spectra at a temperature well below the first excitation energy. The excited-state symmetry is also probed by temperature dependence of the linear dichroism where the high measurement temperatures are on the order of the crystal-field-splitting energies.
Mori, Hiroko*; Uemura, Taiki*; Matsuyama, Hideya*; Abe, Shinichiro; Watanabe, Yukinobu*
no journal, ,
Terrestrial neutron-induced soft error has been recognized as a serious reliability problem for microelectronics. An irradiation testing with spallation neutron beam is suitable for evaluation of terrestrial neutron induced soft error rate (SER). The SER measured in neutron irradiation facility is necessary to compensate since neutron beam spectra obtained each facility is different from terrestrial neutron spectrum reported by JEDEC. We provide SER ratio between JEDEC's and facility's as a function of critical charge so as to compensate SER in various devices. The SER ratio was derived from single event upset (SEU) cross-section calculated by a multi-scale Monte Carlo simulation code system PHYSERD (PHits-HYenexss integrated code System for Effects of Radiation on Device). We performed SER testing using spallation neutron beam at three facilities. The deviation among measured SERs compensated by the SER ratio is less than 5%.
Sugita, Tsuyoshi; Yamazaki, Taiki*; Isaka, Mayu*; Mori, Masanobu*
no journal, ,
Photocatalyst is useful material for air and water purification. The quality of photocatalysts is evaluated by decomposition of targeted chemicals in the batch method. The batch method causes the contamination and the changes of solid-to-liquid ratio. To evaluate of photocatalyst easily and correctly, we developed flow analytical system with liquid chromatography (LC-FAS).
 solution for understanding chemical species of Db
solution for understanding chemical species of DbAdachi, Sadia*; Sueki, Keisuke*; Toyoshima, Atsushi*; Tsukada, Kazuaki; Haba, Hiromitsu*; Komori, Yukiko*; Yokokita, Takuya*; Mori, Taiki*
no journal, ,
Anion exchange behavior of group-5 elements, Nb, Ta, and their pseudo homologue Pa in HF and HF/HNO solution was investigated to understand chemical properties of Db. Adsorption behavior of Db is clearly different from that of Ta and is similar to those of Nb and Pa. In the present study, anion exchange experiments of Nb, Ta and Pa were carried out with HF/HNO
solution was investigated to understand chemical properties of Db. Adsorption behavior of Db is clearly different from that of Ta and is similar to those of Nb and Pa. In the present study, anion exchange experiments of Nb, Ta and Pa were carried out with HF/HNO aqueous solution as basic experiments for estimating the species of Db fluoride complex. Adsorption behavior was investigated by changing the fluoride ion concentration for each HNO
aqueous solution as basic experiments for estimating the species of Db fluoride complex. Adsorption behavior was investigated by changing the fluoride ion concentration for each HNO concentration. Based on the observed distribution coefficients of these elements, we discuss the formation reaction of the anionic fluoride complex and chemical species of Db.
concentration. Based on the observed distribution coefficients of these elements, we discuss the formation reaction of the anionic fluoride complex and chemical species of Db.