Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ichikawa, Tsubasa*; Hakoshima, Hideaki*; Inui, Koji*; Ito, Kosuke*; Matsuda, Ryo*; Mitarai, Kosuke*; Miyamoto, Koichi*; Mizukami, Wataru*; Mizuta, Kaoru*; Mori, Toshio*; et al.
Nature Reviews Physics (Internet), 6(6), p.345 - 347, 2024/06
Times Cited Count:8 Percentile:99.15(Physics, Applied)Mori, Yuichiro*; Kagi, Hiroyuki*; Aoki, Katsutoshi*; Takano, Masahiro*; Kakizawa, Sho*; Sano, Asami; Funakoshi, Kenichi*
Earth and Planetary Science Letters, 634, p.118673_1 - 118673_8, 2024/05
Times Cited Count:1 Percentile:41.61(Geochemistry & Geophysics)To investigate silicon effects on the hydrogen-induced volume expansion of iron, neutron diffraction and X-ray diffraction experiments were conducted to examine hcp-Fe Si
Si under high pressures and high temperatures. Neutron diffraction experiments were performed on the deuterated hcp-Fe
under high pressures and high temperatures. Neutron diffraction experiments were performed on the deuterated hcp-Fe Si
Si at 13.5 GPa and 900 K, and at 12.1 GPa and 300 K. By combining the P-V-T equation of state of hcp-Fe
at 13.5 GPa and 900 K, and at 12.1 GPa and 300 K. By combining the P-V-T equation of state of hcp-Fe Si
Si , present results indicate that the hydrogen-induced volume expansion of hcp-Fe
, present results indicate that the hydrogen-induced volume expansion of hcp-Fe Si
Si is 10% greater than that of pure hcp iron. Using the obtained values, we estimated the hydrogen content that would reproduce the density deficit in the inner core, which was 50% less than that without the effect of silicon. Possible hydrogen content,
is 10% greater than that of pure hcp iron. Using the obtained values, we estimated the hydrogen content that would reproduce the density deficit in the inner core, which was 50% less than that without the effect of silicon. Possible hydrogen content,  , in the inner core and the outer core was calculated to be 0.07 and 0.12-0.15, respectively, when reproducing the density deficit of the inner core with hcp-Fe
, in the inner core and the outer core was calculated to be 0.07 and 0.12-0.15, respectively, when reproducing the density deficit of the inner core with hcp-Fe Si
Si Hx.
Hx.
Takano, Masahiro*; Kagi, Hiroyuki*; Mori, Yuichiro*; Aoki, Katsutoshi*; Kakizawa, Sho*; Sano, Asami; Iizuka-Oku, Riko*; Tsuchiya, Taku*
Journal of Mineralogical and Petrological Sciences (Internet), 119(1), p.240122_1 - 240122_9, 2024/00
Times Cited Count:0 Percentile:0.00(Mineralogy)Hydrogenation of iron sulfide (FeS) under high-pressure and high-temperature conditions has attracted attention because hydrogen and sulfur are promising candidates as light elements in the cores of the Earth and other terrestrial planets. In earlier reports describing the hydrogenation of FeS, the chemical compositions of starting materials were not fully clarified. This study reports in-situ neutron and X-ray diffraction measurements under high-pressure and high-temperature conditions of an Fe-S-H system using a stochiometric Fe1.000S (troilite) as a starting material. The occupancies determined were significantly lower than those reported from earlier studies, indicating that the hydrogenation of FeS can be affected strongly by the stoichiometry of iron sulfide.
Yakushev, A.*; Lens, L.*; D llmann, Ch. E.*; Khuyagbaatar, J.*; J
llmann, Ch. E.*; Khuyagbaatar, J.*; J ger, E.*; Krier, J.*; Runke, J.*; Albers, H. M.*; Asai, Masato; Block, M.*; et al.
ger, E.*; Krier, J.*; Runke, J.*; Albers, H. M.*; Asai, Masato; Block, M.*; et al.
Frontiers in Chemistry (Internet), 10, p.976635_1 - 976635_11, 2022/08
Times Cited Count:19 Percentile:79.78(Chemistry, Multidisciplinary)Flerovium (Fl, element 114) is the heaviest element chemically studied so far. The first chemical experiment on Fl suggested that Fl is a noble-gas-like element, while the second studies suggested that Fl has a volatile-metal-like character. To obtain more reliable conclusion, we performed further experimental studies on Fl adsorption behavior on Si oxide and gold surfaces. The present results suggest that Fl is highly volatile and less reactive than the volatile metal, Hg, but has higher reactivity than the noble gas, Rn.
Iizuka, Riko*; Goto, Hirotada*; Shito, Chikara*; Fukuyama, Ko*; Mori, Yuichiro*; Hattori, Takanori; Sano, Asami; Funakoshi, Kenichi*; Kagi, Hiroyuki*
Scientific Reports (Internet), 11(1), p.12632_1 - 12632_10, 2021/06
Times Cited Count:5 Percentile:33.44(Multidisciplinary Sciences)The Earth's core consist of Fe-Ni alloy with some light elements (H, C, O, Si, S etc.). Hydrogen (H) is the most abundant element in the universe and one of the promising candidates. In this study, we have investigated the effects of sulfur(S) on hydrogenation of iron-hydrous silicate system containing saturated water in the ideal composition of the primitive Earth. We observed a series of phase transitions of Fe, dehydration of the hydrous mineral, and formation of olivine and enstatite with increasing temperature. The FeS formed as the coexisting phase of Fe under high-pressure and temperature condition, but its unit cell volume did not increase, suggesting that FeS is hardly hydrogenated. Recovered samples exhibited that H and S can be incorporated into solid Fe, which lowers the melting temperature as Fe(H )-FeS system. No detection of other light elements (C, O, Si) in solid Fe suggests that they dissolve into molten iron hydride and/or FeS in the later process of Earth's core-mantle differentiation.
)-FeS system. No detection of other light elements (C, O, Si) in solid Fe suggests that they dissolve into molten iron hydride and/or FeS in the later process of Earth's core-mantle differentiation.
Kitazato, Kohei*; Milliken, R. E.*; Iwata, Takahiro*; Abe, Masanao*; Otake, Makiko*; Matsuura, Shuji*; Takagi, Yasuhiko*; Nakamura, Tomoki*; Hiroi, Takahiro*; Matsuoka, Moe*; et al.
Nature Astronomy (Internet), 5(3), p.246 - 250, 2021/03
Times Cited Count:60 Percentile:96.18(Astronomy & Astrophysics)Here we report observations of Ryugu's subsurface material by the Near-Infrared Spectrometer (NIRS3) on the Hayabusa2 spacecraft. Reflectance spectra of excavated material exhibit a hydroxyl (OH) absorption feature that is slightly stronger and peak-shifted compared with that observed for the surface, indicating that space weathering and/or radiative heating have caused subtle spectral changes in the uppermost surface. However, the strength and shape of the OH feature still suggests that the subsurface material experienced heating above 300  C, similar to the surface. In contrast, thermophysical modeling indicates that radiative heating does not increase the temperature above 200
C, similar to the surface. In contrast, thermophysical modeling indicates that radiative heating does not increase the temperature above 200  C at the estimated excavation depth of 1 m, even if the semimajor axis is reduced to 0.344 au. This supports the hypothesis that primary thermal alteration occurred due to radiogenic and/or impact heating on Ryugu's parent body.
C at the estimated excavation depth of 1 m, even if the semimajor axis is reduced to 0.344 au. This supports the hypothesis that primary thermal alteration occurred due to radiogenic and/or impact heating on Ryugu's parent body.
 Si
Si at 14.7 GPa and 800 K
at 14.7 GPa and 800 KMori, Yuichiro*; Kagi, Hiroyuki*; Kakizawa, Sho*; Komatsu, Kazuki*; Shito, Chikara*; Iizuka, Riko*; Aoki, Katsutoshi*; Hattori, Takanori; Sano, Asami; Funakoshi, Kenichi*; et al.
Journal of Mineralogical and Petrological Sciences, 116(6), p.309 - 313, 2021/00
Times Cited Count:2 Percentile:10.53(Mineralogy)The Earth's core is believed to contain some light elements because it is 10% less dense than pure Fe under the corresponding pressure and temperature conditions. Hydrogen, a promising candidate among light elements, has phase relations and physical properties that have been investigated mainly for the Fe-H system. This study specifically examined an Fe-Si-H system using in-situ neutron diffraction experiments to investigate the site occupancy of deuterium of hcp-Fez Si
Si hydride at 14.7 GPa and 800 K. Results of Rietveld refinement indicate hcp-Fe
hydride at 14.7 GPa and 800 K. Results of Rietveld refinement indicate hcp-Fe Si
Si hydride as having deuterium (D) occupancy of 0.24(2) exclusively at the interstitial octahedral site in the hcp lattice. The effect on the site occupancy of D by addition of 2.6 wt% Si into Fe (Fe
hydride as having deuterium (D) occupancy of 0.24(2) exclusively at the interstitial octahedral site in the hcp lattice. The effect on the site occupancy of D by addition of 2.6 wt% Si into Fe (Fe Si
Si ) was negligible compared to results obtained from an earlier study of an Fe-D system (Machida et al., 2019).
) was negligible compared to results obtained from an earlier study of an Fe-D system (Machida et al., 2019).
 and Au surfaces in preparation for chemical investigations on Cn, Nh, and Fl at TASCA
and Au surfaces in preparation for chemical investigations on Cn, Nh, and Fl at TASCALens, L.*; Yakushev, A.*; D llmann, Ch. E.*; Asai, Masato; Ballof, J.*; Block, M.*; David, H. M.*; Despotopulos, J.*; Di Nitto, A.*; Eberhardt, K.*; et al.
llmann, Ch. E.*; Asai, Masato; Ballof, J.*; Block, M.*; David, H. M.*; Despotopulos, J.*; Di Nitto, A.*; Eberhardt, K.*; et al.
Radiochimica Acta, 106(12), p.949 - 962, 2018/12
Times Cited Count:11 Percentile:67.60(Chemistry, Inorganic & Nuclear)Online gas-solid adsorption studies with single atom quantities of Hg, Tl, and Pb on SiO and Au surfaces were carried out using short-lived radioisotopes with half-lives in the range of 4-49 s. This is a model study to measure adsorption enthalpies of superheavy elements Cn, Nh, and Fl. The short-lived isotopes were produced and separated by the gas-filled recoil separator TASCA at GSI. The products were stopped in He gas, and flushed into gas chromatography columns made of Si detectors whose surfaces were covered by SiO
and Au surfaces were carried out using short-lived radioisotopes with half-lives in the range of 4-49 s. This is a model study to measure adsorption enthalpies of superheavy elements Cn, Nh, and Fl. The short-lived isotopes were produced and separated by the gas-filled recoil separator TASCA at GSI. The products were stopped in He gas, and flushed into gas chromatography columns made of Si detectors whose surfaces were covered by SiO or Au. The short-lived Tl and Pb were successfully measured by the Si detectors with the SiO
or Au. The short-lived Tl and Pb were successfully measured by the Si detectors with the SiO surface at room temperature. On the other hand, the Hg did not adsorb on the SiO
surface at room temperature. On the other hand, the Hg did not adsorb on the SiO surface, but adsorbed on the Au surface. The results demonstrated that the adsorption properties of short-lived Hg, Tl, and Pb could be studied with this setup, and that this method is applicable to the experiment for Cn, Nh, and Fl.
surface, but adsorbed on the Au surface. The results demonstrated that the adsorption properties of short-lived Hg, Tl, and Pb could be studied with this setup, and that this method is applicable to the experiment for Cn, Nh, and Fl.
 SO
SO and HF/HCl solutions into toluene with Aliquat336; Sulfate and fluoride complex formation of Mo and W towards chemical studies of seaborgium (Sg)
and HF/HCl solutions into toluene with Aliquat336; Sulfate and fluoride complex formation of Mo and W towards chemical studies of seaborgium (Sg)Toyoshima, Atsushi; Mitsukai, Akina; Tsukada, Kazuaki; Oe, Kazuhiro*; Haba, Hiromitsu*; Komori, Yukiko*; Murakami, Masashi; Kaneya, Yusuke*; Sato, Daisuke*; Asai, Masato; et al.
Journal of Radioanalytical and Nuclear Chemistry, 317(1), p.421 - 430, 2018/07
Times Cited Count:3 Percentile:25.46(Chemistry, Analytical)We have studied extraction behavior of group-6 elements Mo and W to search for suitable conditions for an on-line extraction experiment of their heavier homolog, seaborgium (Sg). Batch-wise extraction of carrier-free radiotracers 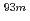 Mo and
Mo and 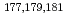 W were carried out from 0.10 - 8.6 M H
W were carried out from 0.10 - 8.6 M H SO
SO and 1.0
and 1.0 10
10 - 5.0 M HF/1.0 M HCl into toluene with a quaternary ammonium compound, Aliquat336. Anionic sulfate complexes of Mo and W with charge - 2 were extracted with Aliquat336 from H
- 5.0 M HF/1.0 M HCl into toluene with a quaternary ammonium compound, Aliquat336. Anionic sulfate complexes of Mo and W with charge - 2 were extracted with Aliquat336 from H SO
SO solutions with concentrations of [H
solutions with concentrations of [H SO
SO ]
]  2 M. In HF/1.0 M HCl, oxyfluoro complexes of Mo and W with charge - 1 were interpreted to be formed and extracted with Aliquat336. From these results, favorable conditions for the extraction of Sg are discussed.
2 M. In HF/1.0 M HCl, oxyfluoro complexes of Mo and W with charge - 1 were interpreted to be formed and extracted with Aliquat336. From these results, favorable conditions for the extraction of Sg are discussed.
Eichler, R.*; Asai, Masato; Brand, H.*; Chiera, N. M.*; Di Nitto, A.*; Dressler, R.*; D llmann, Ch. E.*; Even, J.*; Fangli, F.*; Goetz, M.*; et al.
llmann, Ch. E.*; Even, J.*; Fangli, F.*; Goetz, M.*; et al.
EPJ Web of Conferences, 131, p.07005_1 - 07005_7, 2016/12
Times Cited Count:3 Percentile:71.15(Chemistry, Inorganic & Nuclear)In recent years gas-phase chemical studies assisted by physical pre-separation allowed for the productions and investigations of fragile single molecular species of superheavy elements. The latest highlight is the formation of very volatile hexacarbonyl compound of element 106, Sg(CO) . Following this success, second-generation experiments were performed to measure the first bond dissociation energy between the central metal atom and the surrounding ligand. The method using a tubular decomposition reactor was developed and successfully applied to short-lived Mo(CO)
. Following this success, second-generation experiments were performed to measure the first bond dissociation energy between the central metal atom and the surrounding ligand. The method using a tubular decomposition reactor was developed and successfully applied to short-lived Mo(CO) , W(CO)
, W(CO) , and Sg(CO)
, and Sg(CO) .
.
Oe, Kazuhiro*; Attallah, M. F.*; Asai, Masato; Goto, Naoya*; Gupta, N. S.*; Haba, Hiromitsu*; Huang, M.*; Kanaya, Jumpei*; Kaneya, Yusuke*; Kasamatsu, Yoshitaka*; et al.
Journal of Radioanalytical and Nuclear Chemistry, 303(2), p.1317 - 1320, 2015/02
Times Cited Count:10 Percentile:60.46(Chemistry, Analytical)A new technique for continuous dissolution of nuclear reaction products transported by a gas-jet system was developed for superheavy element (SHE) chemistry. In this technique, a hydrophobic membrane is utilized to separate an aqueous phase from the gas phase. With this technique, the dissolution efficiencies of short-lived radionuclides of 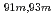 Mo and
Mo and 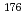 W were measured. Yields of more than 80% were observed for short-lived radionuclides at aqueous-phase flow rates of 0.1-0.4 mL/s. The gas flow-rate had no influence on the dissolution efficiency within the studied flow range of 1.0-2.0 L/min. These results show that this technique is applicable for on-line chemical studies of SHEs in the liquid phase.
W were measured. Yields of more than 80% were observed for short-lived radionuclides at aqueous-phase flow rates of 0.1-0.4 mL/s. The gas flow-rate had no influence on the dissolution efficiency within the studied flow range of 1.0-2.0 L/min. These results show that this technique is applicable for on-line chemical studies of SHEs in the liquid phase.
 SO
SO /HNO
/HNO mixed solution
mixed solutionLi, Z.*; Toyoshima, Atsushi; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Sato, Nozomi; Kikuchi, Takahiro; Nagame, Yuichiro; Sch del, M.; Pershina, V.*; et al.
del, M.; Pershina, V.*; et al.
Radiochimica Acta, 100(3), p.157 - 164, 2012/03
Times Cited Count:14 Percentile:68.81(Chemistry, Inorganic & Nuclear)Ishii, Yasuo; Toyoshima, Atsushi; Tsukada, Kazuaki; Asai, Masato; Li, Z.*; Nagame, Yuichiro; Miyashita, Sunao*; Mori, Tomotaka*; Suganuma, Hideo*; Haba, Hiromitsu*; et al.
Bulletin of the Chemical Society of Japan, 84(9), p.903 - 911, 2011/09
Times Cited Count:18 Percentile:49.57(Chemistry, Multidisciplinary)The cation-exchange behavior of element 104, rutherfordium (Rf), was investigated together with its lighter group-4 homologues Zr and Hf, and the tetravalent pseudo-homologue Th in HF/HNO mixed solution. The results demonstrate that distribution coefficients (
mixed solution. The results demonstrate that distribution coefficients (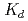 ) of Rf in HF/0.10 M HNO
) of Rf in HF/0.10 M HNO decrease with increasing concentration of the fluoride ion [F
decrease with increasing concentration of the fluoride ion [F ], indicating the consecutive formation of fluorido complexes of Rf. We also measured the
], indicating the consecutive formation of fluorido complexes of Rf. We also measured the 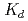 values of Rf and the homologues as a function of the hydrogen ion concentration [H
values of Rf and the homologues as a function of the hydrogen ion concentration [H ]. The log
]. The log 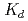 values decrease linearly with an increase of log [H
values decrease linearly with an increase of log [H ] with slopes between -2.1 and -2.5. This indicates that these elements are likely to form the same chemical compounds: mixture of [MF]
] with slopes between -2.1 and -2.5. This indicates that these elements are likely to form the same chemical compounds: mixture of [MF] and [MF
and [MF ]
] (M = Rf, Zr, Hf and Th) in the studied solution. It is also ascertained that sequence in the fluoride complex formation is Zr
(M = Rf, Zr, Hf and Th) in the studied solution. It is also ascertained that sequence in the fluoride complex formation is Zr  Hf
Hf  Rf
Rf  Th.
Th.
 electronic states in CeIrSi
electronic states in CeIrSi studied by angle-resolved Ce 3
studied by angle-resolved Ce 3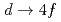 resonance photoemission spectroscopy
resonance photoemission spectroscopyOkochi, Takuo*; Toshimitsu, Takafumi*; Yamagami, Hiroshi; Fujimori, Shinichi; Yasui, Akira; Takeda, Yukiharu; Okane, Tetsuo; Saito, Yuji; Fujimori, Atsushi; Miyauchi, Yuichiro*; et al.
Journal of the Physical Society of Japan, 78(8), p.084802_1 - 084802_6, 2009/08
Times Cited Count:10 Percentile:53.51(Physics, Multidisciplinary)We have applied angle-resolved Ce 3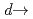 4
4 resonance photoemission spectroscopy to the non-centrosymmetric pressure-induced superconductor CeIrSi
resonance photoemission spectroscopy to the non-centrosymmetric pressure-induced superconductor CeIrSi and obtained the 4
and obtained the 4 band-structure and Fermi surfaces. We have found that the Ce 4
band-structure and Fermi surfaces. We have found that the Ce 4 states are located mainly near the Fermi level and that the photoemission intensity derived from the dispersive conduction bands across the Fermi level shows considerable resonant enhancement. In addition, the band structure and Fermi surfaces of CeIrSi
states are located mainly near the Fermi level and that the photoemission intensity derived from the dispersive conduction bands across the Fermi level shows considerable resonant enhancement. In addition, the band structure and Fermi surfaces of CeIrSi are different from those of the non-
are different from those of the non- reference compound, LaIrSi
reference compound, LaIrSi and the difference is well explained by the band structure calculated within the local density approximation (LDA). These results strongly suggest that the Ce 4
and the difference is well explained by the band structure calculated within the local density approximation (LDA). These results strongly suggest that the Ce 4 electrons in CeIrSi
electrons in CeIrSi are well hybridized with conduction bands and form itinerant electronic states.
are well hybridized with conduction bands and form itinerant electronic states.
Ishii, Yasuo; Toyoshima, Atsushi; Tsukada, Kazuaki; Asai, Masato; Tome, Hayato; Nishinaka, Ichiro; Nagame, Yuichiro; Miyashita, Sunao*; Mori, Tomotaka*; Suganuma, Hideo*; et al.
Chemistry Letters, 37(3), p.288 - 289, 2008/03
Times Cited Count:21 Percentile:55.31(Chemistry, Multidisciplinary)We have investigated cation-exchange behavior of Rf together with the lighter homologues of the group-4 elements Zr and Hf, and the tetravalent pseudo-homologue Th, in HF/HNO solution using Automated Ion exchange separation apparatus coupled with the Detection system for Alpha spectroscopy (AIDA). The
solution using Automated Ion exchange separation apparatus coupled with the Detection system for Alpha spectroscopy (AIDA). The 
 values of Zr, Hf, Th and Rf in HF/0.1 M HNO
values of Zr, Hf, Th and Rf in HF/0.1 M HNO were decreased with increasing the concentration of the fluoride ion [F
were decreased with increasing the concentration of the fluoride ion [F ], indicating the formation of the fluoride complexes. The sequence of the fluoride complexation strength is Zr
], indicating the formation of the fluoride complexes. The sequence of the fluoride complexation strength is Zr  Hf
Hf  Rf
Rf  Th.
Th.
Hanawa, Satoshi; Sato, Tomonori; Mori, Yuichiro; Ogiyanagi, Jin; Kaji, Yoshiyuki; Uchida, Shunsuke
Journal of Power and Energy Systems (Internet), 1(2), p.123 - 133, 2007/00
In order to evaluate the water chemistry in the irradiation field during IASCC irradiation test, a water radiolysis code for IASCC irradiation loop system was developed. In the water radiolysis code, a multiple node model was introduced since the irradiation loop system has a wide rage temperature distribution as well as the dose distribution. To investigate the applicability of developed water radiolysis code, water chemistry at the water sampling point of the irradiation loop system was measured and compared with analytical results under several water chemistry conditions. Further, water chemistry distribution in the in-pile region as well as in the out-pile region was calculated by the developed water radiolysis code.
Hanawa, Satoshi; Sato, Tomonori; Mori, Yuichiro; Ogiyanagi, Jin; Kaji, Yoshiyuki; Uchida, Shunsuke*
Proceedings of 14th International Conference on Nuclear Engineering (ICONE-14) (CD-ROM), 9 Pages, 2006/07
no abstracts in English
Hanawa, Satoshi; Ogiyanagi, Jin; Mori, Yuichiro*; Saito, Junichi; Tsukada, Takashi
JAEA-Conf 2006-003, p.350 - 357, 2006/05
no abstracts in English
Mori, Yuichiro*; Ide, Hiroshi; Nabeya, Hideaki; Tsukada, Takashi
JAERI-Tech 2002-003, 32 Pages, 2002/02
In relation to the aging of Light Water Reactor (LWR), the Irradiation Assisted Stress Corrosion Cracking (IASCC) has been regarded as a significant and urgent issue for the reliability of in-core components of LWR, and the irradiation research on the IASCC is now under schedule. With the progress of the irradiation research on reactor materials, well-controlled environment conditions during irradiation testing are required. Especially for irradiation testing of IASCC studies, water chemistry control is essential in addition to the control of neutron fluence and irradiation temperature.According to these requirements, at the Japan Atomic Energy Research Institute (JAERI), an irradiation testing facility that simulates in-core environment of Boiling Water Reactor (BWR) has been designed to be installed in the Japan Materials Testing Reactor (JMTR). This facility is composed of the Saturated Temperature Capsules (SATCAP) that are installed into the JMTR's core to irradiate material specimens, the Water Control Unit that is able to supply high-temperature and high-pressure chemical controlled water to SATCAP, and other components.This report describes the design study of water chemistry control system of the Water Control Unit. The design work has been performed in the fiscal year 1999.
Kanno, Masaru; Nabeya, Hideaki; Mori, Yuichiro*; Matsui, Yoshinori; Tobita, Masahiro*; Ide, Hiroshi; Itabashi, Yukio; Komori, Yoshihiro; Tsukada, Takashi; Tsuji, Hirokazu
JAERI-Tech 2001-080, 57 Pages, 2001/12
no abstracts in English