Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Toda, Taro*; Maruyama, Takehiro*; Moritani, Kimikazu*; Moriyama, Hirotake*; Hayashi, Hirokazu
Denki Kagaku Oyobi Kogyo Butsuri Kagaku, 77(8), p.649 - 651, 2009/08
Times Cited Count:8 Percentile:19.4(Electrochemistry)The distribution coefficients of Am and Ce were measured in the eutectic LiCl-KCl/liquid Ga system at 773K. By using ZrCl as the oxide ion scavenger in order to avoid the formation of such oxychlorides as MO
as the oxide ion scavenger in order to avoid the formation of such oxychlorides as MO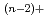 , the effect of oxide ion concentration was well controlled on the distribution coefficients of Am and Ce. The separation factor between Am and Ce was then obtained to be about 100. By comparing the present value with the other experimental and the predicted ones, it was confirmed that the Ga system was more selective than the Bi and Cd system.
, the effect of oxide ion concentration was well controlled on the distribution coefficients of Am and Ce. The separation factor between Am and Ce was then obtained to be about 100. By comparing the present value with the other experimental and the predicted ones, it was confirmed that the Ga system was more selective than the Bi and Cd system.
Moriyama, Hirotake*; Moritani, Kimikazu*; Toda, Taro*; Hayashi, Hirokazu
Radiochimica Acta, 97(4-5), p.233 - 236, 2009/05
Times Cited Count:1 Percentile:10.22(Chemistry, Inorganic & Nuclear)The distribution coefficients of Am, Ce, and Eu between the salt and metal phases were measured at 1073 K in a reductive extraction system of equimolar NaCl-KCl melt and liquid Ga, which were expected to be higher from a thermodynamic point of view. By changing some solute concentrations, it was observed that the distribution coefficients were much dependent on the oxide ion concentration in the system, possibly due to the formation of such compounds as AmO and CeO
and CeO . Also, in some runs, the mass balance of Am was affected possibly by the formation and precipitation of its oxychloride and/or oxide. By taking the observations into consideration, the separation factor between Am and Ce was obtained to be about 30 as a lower limit in the present system.
. Also, in some runs, the mass balance of Am was affected possibly by the formation and precipitation of its oxychloride and/or oxide. By taking the observations into consideration, the separation factor between Am and Ce was obtained to be about 30 as a lower limit in the present system.
Toda, Taro*; Maruyama, Takehiro*; Moritani, Kimikazu*; Moriyama, Hirotake*; Hayashi, Hirokazu
Journal of Nuclear Science and Technology, 46(1), p.18 - 25, 2009/01
Times Cited Count:41 Percentile:92.56(Nuclear Science & Technology)The excess thermodynamic quantities of lanthanides and actinides in molten salts and liquid metals were studied for reductive extraction of minor actinides. The excess enthalpies and entropies of those elements in the molten chloride phase were found to be correlated with the ionic radii of metal ions possibly due to complex formation. In the liquid metal phase, on the other hand, the excess enthalpies were explained with Miedema's atomistic model and the excess entropies were explained with the vibrational entropy due to alloy formation. Using these correlations and models, some missing values of the excess thermodynamic quantities were evaluated and the separation factors of minor actinides from lanthanides were calculated in different reductive extraction systems. The higher separation factors were obtained in the system using aluminum or gallium than in the system using bismuth or cadmium as the liquid metal phase.
Toda, Taro*; Maruyama, Takehiro*; Moritani, Kimikazu*; Moriyama, Hirotake*; Hayashi, Hirokazu
Proceedings of 2008 Joint Symposium on Molten Salts (USB Flash Drive), p.933 - 938, 2008/10
The distribution coefficients of Am and Ce were measured in the LiCl-KCl/Ga system at 773 K. By using ZrCl as the oxide ion scavenger in order to avoid the formation of such oxychlorides as CeO
as the oxide ion scavenger in order to avoid the formation of such oxychlorides as CeO and AmO
and AmO , the effect of oxide ion concentration was well controlled on the distribution coefficients of Am and Ce. The separation factor between Am and Ce was then obtained to be about 100. By comparing the present value with the other experimental and the predicted ones, it was confirmed that solvent metals were ordered from the most selective to the less selective one as Al
, the effect of oxide ion concentration was well controlled on the distribution coefficients of Am and Ce. The separation factor between Am and Ce was then obtained to be about 100. By comparing the present value with the other experimental and the predicted ones, it was confirmed that solvent metals were ordered from the most selective to the less selective one as Al Ga
Ga Bi
Bi Cd.
Cd.