Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 /Si(001) system observed by SiO mass spectrometry and synchrotron radiation photoemission spectroscopy"
/Si(001) system observed by SiO mass spectrometry and synchrotron radiation photoemission spectroscopy"Teraoka, Yuden; Moritani, Kosuke*; Yoshigoe, Akitaka
Applied Surface Science, 343, P. 213, 2015/07
Times Cited Count:0 Percentile:0.00(Chemistry, Physical) molecular beams under 1000 K
molecular beams under 1000 KTeraoka, Yuden; Yoshigoe, Akitaka; Moritani, Kosuke*
Japanese Journal of Applied Physics, 54(3), P. 039202_1, 2015/03
Times Cited Count:0 Percentile:0.00(Physics, Applied) molecular beams
molecular beamsOkada, Michio*; Moritani, Kosuke*; Vattuone, L.*; Savio, L.*; Teraoka, Yuden; Kasai, Toshio*; Rocca, M.*
Metal Oxide Nanostructures and Their Applications, 1, p.205 - 237, 2010/03
The use of hyperthermal O molecular beams may improve the quality of thin film growth, for example, for organic films, and allow the production of oxide layers at lower crystal temperatures, avoiding contamination problems and reducing film defects. Collision-induced absorption and local heating of the substrate were shown to be indeed effective in inducing oxide nucleation, opening up new possibilities for the production of nanostructured metal oxides. Herein we offer an overview on recent detailed studies of oxygen adsorption and of the initial stages of Cu
molecular beams may improve the quality of thin film growth, for example, for organic films, and allow the production of oxide layers at lower crystal temperatures, avoiding contamination problems and reducing film defects. Collision-induced absorption and local heating of the substrate were shown to be indeed effective in inducing oxide nucleation, opening up new possibilities for the production of nanostructured metal oxides. Herein we offer an overview on recent detailed studies of oxygen adsorption and of the initial stages of Cu O and CuO formation on low Miller index and vicinal Cu surfaces. We introduce the hyperthermal molecular beam technique and give some details on the experimental apparatuses. We discuss the available data for Cu(100), Cu(410) and Cu(110) and Cu(111), respectively.
O and CuO formation on low Miller index and vicinal Cu surfaces. We introduce the hyperthermal molecular beam technique and give some details on the experimental apparatuses. We discuss the available data for Cu(100), Cu(410) and Cu(110) and Cu(111), respectively.
Moritani, Kosuke*; Okada, Michio*; Teraoka, Yuden; Yoshigoe, Akitaka; Kasai, Toshio*
Journal of Physical Chemistry A, 113(52), p.15217 - 15222, 2009/10
Times Cited Count:11 Percentile:34.34(Chemistry, Physical)Oxygen adsorption and subsequent oxide formation on Cu(110) using a hyperthermal oxygen molecular beam (HOMB) has been investigated using SR-XPS. The O-uptake curves, which were determined from the evolution of O-1s peaks, indicate that simple Langmuir type kinetics can describe dissociative adsorption of O with an incident energy (Ei) below 0.5eV under oxygen coverage of 0.5 ML. The reaction order dependence on Ei implies two competing dissociation mechanisms, trapping-mediated and directly-activated adsorption. Oxidation at oxygen coverage larger than 0.5 ML proceeds rather effectively using highly energetic HOMB at Ei larger than 1.0 eV. The surface Cu
with an incident energy (Ei) below 0.5eV under oxygen coverage of 0.5 ML. The reaction order dependence on Ei implies two competing dissociation mechanisms, trapping-mediated and directly-activated adsorption. Oxidation at oxygen coverage larger than 0.5 ML proceeds rather effectively using highly energetic HOMB at Ei larger than 1.0 eV. The surface Cu O formed with highly-energetic HOMB incidence decomposes with desorbing subsurface oxygen even at room temperature. This indicates that HOMB can induce a meta stable surface structure that cannot be produced in the thermal equilibrium process.
O formed with highly-energetic HOMB incidence decomposes with desorbing subsurface oxygen even at room temperature. This indicates that HOMB can induce a meta stable surface structure that cannot be produced in the thermal equilibrium process.
Teraoka, Yuden; Yoshigoe, Akitaka; Moritani, Kosuke*
Electrical Engineering in Japan, 164(3), p.60 - 68, 2008/08
Times Cited Count:3 Percentile:25.63(Engineering, Electrical & Electronic)Oxidation reactions at Si(001) surfaces have been studied via real-time in-situ photoemission spectroscopy with synchrotron radiation for chemical bonding states of Si and O atoms and mass spectrometry for desorption of SiO molecules with supersonic O molecular beams in a temperature region from 900 K to 1300 K. In this study, simultaneous measurements of Si 2p photoemission spectra and SiO desorption yield revealed that the decrease of SiO correlated with the increase of Si
molecular beams in a temperature region from 900 K to 1300 K. In this study, simultaneous measurements of Si 2p photoemission spectra and SiO desorption yield revealed that the decrease of SiO correlated with the increase of Si component, and the SiO desorption was terminated at the oxide thickness of 0.22 nm. These facts suggest that the SiO desorption takes place at the topmost Si dimers and a precursor for SiO desorption is so called a T site, in which O atoms are bonding with the dangling bonds of the dimers. Consequently, M1 and M2 in the Dual-Oxide-Species (DOS) model has been clarified to be a T site and an Si
component, and the SiO desorption was terminated at the oxide thickness of 0.22 nm. These facts suggest that the SiO desorption takes place at the topmost Si dimers and a precursor for SiO desorption is so called a T site, in which O atoms are bonding with the dangling bonds of the dimers. Consequently, M1 and M2 in the Dual-Oxide-Species (DOS) model has been clarified to be a T site and an Si state, respectively.
state, respectively.
Moritani, Kosuke*; Okada, Michio*; Teraoka, Yuden; Yoshigoe, Akitaka; Kasai, Toshio*
Journal of Physical Chemistry C, 112(23), p.8662 - 8667, 2008/06
Times Cited Count:34 Percentile:67.85(Chemistry, Physical)This paper reports a study on the reconstruction at Cu(111) surface induced by a hyperthermal oxygen molecular beam (HOMB) at room temperature. The HOMB incidence at translational energies larger than 0.5 eV induced surface reconstruction in the oxygen coverage larger than 0.27 ML. On the other hand, long-range-ordered structures were not formed even at the oxygen coverage of 0.4 ML for the backfilling of thermal O at room temperature. The O1s XPS peak for the HOMB incidence at room temperature was resolved into two components, 529.4 and 528.9 eV, at the oxygen coverage larger than 0.27 ML, which can be assigned to the O atoms occupying the threefold hollow sites on the unreconstructed Cu(111) surface and four-coordinated sites on the reconstructed structure, respectively. Annealing the reconstructed surface at 620 K decreased the oxygen coverage to 0.27 ML and induced so-called "29" superstructure.
at room temperature. The O1s XPS peak for the HOMB incidence at room temperature was resolved into two components, 529.4 and 528.9 eV, at the oxygen coverage larger than 0.27 ML, which can be assigned to the O atoms occupying the threefold hollow sites on the unreconstructed Cu(111) surface and four-coordinated sites on the reconstructed structure, respectively. Annealing the reconstructed surface at 620 K decreased the oxygen coverage to 0.27 ML and induced so-called "29" superstructure.
Hashinokuchi, Michihiro*; Okada, Michio*; Ito, Hironori*; Kasai, Toshio*; Moritani, Kosuke*; Teraoka, Yuden
Physical Review Letters, 100(25), p.256104_1 - 256104_4, 2008/06
Times Cited Count:16 Percentile:64.93(Physics, Multidisciplinary)We report results of studies on surface-temperature dependence of steric effects in dissociative adsorption processes of NO molecules on Si(111)-(7 7) surface by means of X-ray photoemission spectroscopy. Data presented here show that the reactive sticking probability for N-end collisions is larger than that in O-end collisions at an incident energy of 58 meV. Furthermore, this steric preference is quite sensitive to the surface temperature and the surface coverage. These facts reveal that transient surface trapping of NO molecules in a shallow precursor state plays a key role in the initial step of the NO decomposition on the Si(111)-(7
7) surface by means of X-ray photoemission spectroscopy. Data presented here show that the reactive sticking probability for N-end collisions is larger than that in O-end collisions at an incident energy of 58 meV. Furthermore, this steric preference is quite sensitive to the surface temperature and the surface coverage. These facts reveal that transient surface trapping of NO molecules in a shallow precursor state plays a key role in the initial step of the NO decomposition on the Si(111)-(7 7) surface.
7) surface.
Okada, Michio*; Hashinokuchi, Michihiro*; Moritani, Kosuke*; Kasai, Toshio*; Teraoka, Yuden
Japanese Journal of Applied Physics, 47(5), p.3686 - 3691, 2008/05
Times Cited Count:5 Percentile:22.08(Physics, Applied)An oriented-molecular-beam technique based on the Stark effect of a molecule in an inhomogeneous hexapole electrostatic field is a potential tool for stereochemical control of surface reactions. This technique allows us to select a specific rotational quantum state and also an orientation of a reagent molecule. We have developed a new UHV-compatible oriented-molecular-beam machine. This apparatus is equipped with components for X-ray photoemission spectroscopy (XPS) in order to detect surface-reaction products. In the dissociative adsorption of NO on an Si(111) surface, we found a steric effect in the reactivity by monitoring the products on the surface with the new machine. The N-end collision is more reactive than the O-end collision at an incident energy of 58 meV. To our knowledge, this is the first measurement of the steric effect appearing in the reaction products on the surface.
 7) surface
7) surfaceHashinokuchi, Michihiro*; Ito, Hironori*; Teraoka, Yuden; Moritani, Kosuke*; Okada, Michio*; Kasai, Toshio*
Japanese Journal of Applied Physics, 47(3), p.1672 - 1676, 2008/03
Times Cited Count:4 Percentile:18.26(Physics, Applied)Dissociative adsorption of nitric oxide (NO) on Si(111)-7 7 surface between 330-600 K was investigated using X-ray photoelectron spectroscopy. The uptake curves of both N and O atoms as a function of NO dose revealed a prominent temperature dependence of dissociative adsorption of NO on an Si(111)-7
7 surface between 330-600 K was investigated using X-ray photoelectron spectroscopy. The uptake curves of both N and O atoms as a function of NO dose revealed a prominent temperature dependence of dissociative adsorption of NO on an Si(111)-7 7 surface. The decrease in the rates of dissociative adsorption of NO with increasing surface temperature suggested existence of a precursor state. Additionally, the N/O ratio on the surface changed from 1.0 at 330 K to 1.2 at 600 K. This increasing N/O ratio with increasing surface temperature suggests that an additional reaction path opens at higher surface temperature.
7 surface. The decrease in the rates of dissociative adsorption of NO with increasing surface temperature suggested existence of a precursor state. Additionally, the N/O ratio on the surface changed from 1.0 at 330 K to 1.2 at 600 K. This increasing N/O ratio with increasing surface temperature suggests that an additional reaction path opens at higher surface temperature.
 O formation via hyperthermal O
O formation via hyperthermal O adsorption at Cu(410)
adsorption at Cu(410)Okada, Michio*; Vattuone, L.*; Gerbi, A.*; Savio, L.*; Rocca, M.*; Moritani, Kosuke*; Teraoka, Yuden; Kasai, Toshio*
Journal of Physical Chemistry C, 111(46), p.17340 - 17345, 2007/11
Times Cited Count:18 Percentile:49.84(Chemistry, Physical)We report a study on the oxidation process of Cu(410) using high-resolution electron energy loss spectroscopy and X-ray photoemission spectroscopy using synchrotron radiation. We demonstrate that a hyperthermal O molecular beam (HOMB) is an efficient tool to fabricate Cu
molecular beam (HOMB) is an efficient tool to fabricate Cu O thin film also at room temperature. The efficiency of the Cu
O thin film also at room temperature. The efficiency of the Cu O formation in the initial stages depends on the angle of incidence of HOMB. Step roughening, acting as a source of mobile precursor Cu adatoms and opening channels for bulk diffusion of the O atoms, is a key feature in determining the Cu
O formation in the initial stages depends on the angle of incidence of HOMB. Step roughening, acting as a source of mobile precursor Cu adatoms and opening channels for bulk diffusion of the O atoms, is a key feature in determining the Cu O formation rate.
O formation rate.
Okada, Michio*; Vattuone, L.*; Moritani, Kosuke*; Savio, L.*; Teraoka, Yuden; Kasai, Toshio*; Rocca, M.*
Journal of Physics; Condensed Matter, 19(30), p.305022_1 - 305022_7, 2007/08
Times Cited Count:8 Percentile:37.62(Physics, Condensed Matter)We studied the oxidation of Cu(410) for thermal O exposure and using High-resolution Electron Energy-loss Spectroscopy. Cu
exposure and using High-resolution Electron Energy-loss Spectroscopy. Cu O is identified by loss peaks at 19 meV and 79 meV. By monitoring the intensity of the latter, we find that Cu
O is identified by loss peaks at 19 meV and 79 meV. By monitoring the intensity of the latter, we find that Cu O formation depends strongly on surface temperature and on O
O formation depends strongly on surface temperature and on O pressure and is kinetically limited by the impinging O
pressure and is kinetically limited by the impinging O flux. Thermally activated step roughening, leading to detachment of Cu adatoms from the step edge, acts as a source of mobile Cu atoms allowing for subsequent nucleation of Cu
flux. Thermally activated step roughening, leading to detachment of Cu adatoms from the step edge, acts as a source of mobile Cu atoms allowing for subsequent nucleation of Cu O patches.
O patches.
 on Cu surfaces
on Cu surfacesMoritani, Kosuke*; Tsuda, Muneyuki*; Teraoka, Yuden; Okada, Michio*; Yoshigoe, Akitaka; Fukuyama, Tetsuya*; Kasai, Toshio*; Kasai, Hideaki*
Journal of Physical Chemistry C, 111(27), p.9961 - 9967, 2007/07
Times Cited Count:16 Percentile:46.61(Chemistry, Physical)We report an X-ray photoemission study of the dissociative adsorption of O at Cu(111), (001), and (110) surfaces with an O
at Cu(111), (001), and (110) surfaces with an O molecular beam generated with a variable temperature nozzle. The O-uptake curves, which are produced from precisely measured O-1s peaks, indicate that the dissociative absorption is enhanced as the nozzle temperature is increased up to 1000 K for the normal incidence of O
molecular beam generated with a variable temperature nozzle. The O-uptake curves, which are produced from precisely measured O-1s peaks, indicate that the dissociative absorption is enhanced as the nozzle temperature is increased up to 1000 K for the normal incidence of O at a kinetic energy of 0.5 eV. However, further increasing the nozzle temperature to 1400 K reduces the probability of dissociativeadsorption. These results suggest that vibrational excitations of incident O
at a kinetic energy of 0.5 eV. However, further increasing the nozzle temperature to 1400 K reduces the probability of dissociativeadsorption. These results suggest that vibrational excitations of incident O assist dissociative adsorption while rotational excitations hinder it.
assist dissociative adsorption while rotational excitations hinder it.
 molecular beam
molecular beamOkada, Michio*; Vattuone, L.*; Moritani, Kosuke*; Savio, L.*; Teraoka, Yuden; Kasai, Toshio*; Rocca, M.*
Physical Review B, 75(23), p.233413_1 - 233413_4, 2007/06
Times Cited Count:35 Percentile:76.52(Materials Science, Multidisciplinary)We studied the oxidation of Cu(410) using X-ray photoemission spectroscopy performed with synchrotron radiation. We demonstrate that a hyperthermal O molecular beam is an efficient tool to fabricate Cu oxide thin films at room temperature (RT) and even lower temperatures. At RT, mainly Cu
molecular beam is an efficient tool to fabricate Cu oxide thin films at room temperature (RT) and even lower temperatures. At RT, mainly Cu O forms. At around 100 K, CuO nucleation also takes place; this is noteworthy, since this moiety is usually produced only at much higher
O forms. At around 100 K, CuO nucleation also takes place; this is noteworthy, since this moiety is usually produced only at much higher  and ambient pressure.
and ambient pressure.
 beam
beamOgawa, Shuichi*; Takakuwa, Yuji*; Ishizuka, Shinji*; Yoshigoe, Akitaka; Teraoka, Yuden; Moritani, Kosuke*; Mizuno, Yoshiyuki*
Denki Gakkai Rombunshi, C, 127(2), p.140 - 145, 2007/02
The initial sticking probability of O molecule on a Ti(0001)-1
molecule on a Ti(0001)-1 1 surface at room temperature was measured as a function of translational kinetic energy by real-time photoelectron spectroscopy. The O 1s photoelectron spectra can be fitted well with three components A, B, and C, where the chemical shift of component B and C are +0.7 and +1.6 eV relative to the binding energy of component A (528.8 eV). Upon exposing to the O
1 surface at room temperature was measured as a function of translational kinetic energy by real-time photoelectron spectroscopy. The O 1s photoelectron spectra can be fitted well with three components A, B, and C, where the chemical shift of component B and C are +0.7 and +1.6 eV relative to the binding energy of component A (528.8 eV). Upon exposing to the O beam, component A and C appear dominantly and component B grows with an incubation time, indicating that two kinds of chemical adsorption states are concerned with dissociative adsorption of O
beam, component A and C appear dominantly and component B grows with an incubation time, indicating that two kinds of chemical adsorption states are concerned with dissociative adsorption of O molecule at the initial stage. The incident energy dependence of initial sticking probability shows quite different behaviours between component A and C: initial sticking probability of component C decreases monotonously with incident energy and is almost constant above 0.6 eV, while initial sticking probability of component A shows a rapid decrease followed by a gradual increase with a minimum at 0.5 eV and then decreases with two small maxima at 0.9 and 1.8 eV. The observed incident energy dependence of initial sticking probability for component A and C are discussed in terms of a trapping-mediated dissociative adsorption and a direct dissociative adsorption process.
molecule at the initial stage. The incident energy dependence of initial sticking probability shows quite different behaviours between component A and C: initial sticking probability of component C decreases monotonously with incident energy and is almost constant above 0.6 eV, while initial sticking probability of component A shows a rapid decrease followed by a gradual increase with a minimum at 0.5 eV and then decreases with two small maxima at 0.9 and 1.8 eV. The observed incident energy dependence of initial sticking probability for component A and C are discussed in terms of a trapping-mediated dissociative adsorption and a direct dissociative adsorption process.
Teraoka, Yuden; Yoshigoe, Akitaka; Moritani, Kosuke*
Denki Gakkai Rombunshi, C, 127(2), p.133 - 139, 2007/02
Oxidation reactions at Si(001) surfaces has been studied via real-time in-situ photoemission spectroscopy for chemical bonding states of Si and O atoms, and mass spectrometry for desorbing SiO molecules with synchrotron radiation and supersonic O molecular beams in the temperature range from 900K to 1300K. With increasing an incident energy in the temperature range from 900K to 1000K, the SiO desorption yield decreased. In that case, the time evolution of an Si 2p photoemission spectrum showed that SiO
molecular beams in the temperature range from 900K to 1300K. With increasing an incident energy in the temperature range from 900K to 1000K, the SiO desorption yield decreased. In that case, the time evolution of an Si 2p photoemission spectrum showed that SiO structure at the surface was easily formed by the action of larger incident energy and the increased SiO
structure at the surface was easily formed by the action of larger incident energy and the increased SiO coverage correlated with the decreased SiO desorption yield. Coincidence measurements of Si 2p photoemission spectra and SiO desorption yield revealed that the decrease of SiO correlated with the increase of Si
coverage correlated with the decreased SiO desorption yield. Coincidence measurements of Si 2p photoemission spectra and SiO desorption yield revealed that the decrease of SiO correlated with the increase of Si component, and the SiO desorption was terminated at the oxide thickness of 0.22nm. These facts indicate that the SiO desorption take place at the topmost Si dimmers and its precursor is so called T site, in which O atoms are bonding to the dangling bonds of the dimmers. Consequently, M1 and M2 in the Dual-Oxide-Species (DOS) model were clarified to be T sites and Si
component, and the SiO desorption was terminated at the oxide thickness of 0.22nm. These facts indicate that the SiO desorption take place at the topmost Si dimmers and its precursor is so called T site, in which O atoms are bonding to the dangling bonds of the dimmers. Consequently, M1 and M2 in the Dual-Oxide-Species (DOS) model were clarified to be T sites and Si states, respectively.
states, respectively.
 Au(100) using hyperthermal O
Au(100) using hyperthermal O molecular beam
molecular beamOkada, Michio*; Hashinokuchi, Michihiro*; Fukuoka, Masayuki*; Kasai, Toshio*; Moritani, Kosuke*; Teraoka, Yuden
Applied Physics Letters, 89(20), p.201912_1 - 201912_3, 2006/11
Times Cited Count:26 Percentile:65.15(Physics, Applied)Oxidation of Cu Au(100) using a hyperthermal O
Au(100) using a hyperthermal O molecular beam (HOMB) was investigated by X-ray photoemission spectroscopy in conjunction with a synchrotron light source. From the incident energy dependence of the O-uptake curve, it was determined that the dissociative adsorption of O
molecular beam (HOMB) was investigated by X-ray photoemission spectroscopy in conjunction with a synchrotron light source. From the incident energy dependence of the O-uptake curve, it was determined that the dissociative adsorption of O implies a higher activation barrier and therefore less reactivity due to the Au alloying. The dissociative adsorption progresses with the Cu segregation on the surface. No prominent growth of Cu
implies a higher activation barrier and therefore less reactivity due to the Au alloying. The dissociative adsorption progresses with the Cu segregation on the surface. No prominent growth of Cu O even for 2 eV HOMB suggests that the Au-alloying of Cu can serve as a protective layer against further oxidation into the bulk.
O even for 2 eV HOMB suggests that the Au-alloying of Cu can serve as a protective layer against further oxidation into the bulk.
 Au surfaces with a hyperthermal O
Au surfaces with a hyperthermal O molecular beam
molecular beamOkada, Michio*; Moritani, Kosuke; Fukuyama, Tetsuya*; Mizutani, Hironori*; Yoshigoe, Akitaka; Teraoka, Yuden; Kasai, Toshio*
Surface Science, 600(18), p.4228 - 4232, 2006/09
Times Cited Count:20 Percentile:63.36(Chemistry, Physical)Dissociative adsorption of hyperthermal O molecules on Cu
molecules on Cu Au(100) was investigated by X-ray photoemission spectroscopy in conjunction with a synchrotron light source. Comparing the O-uptake curve on Cu
Au(100) was investigated by X-ray photoemission spectroscopy in conjunction with a synchrotron light source. Comparing the O-uptake curve on Cu Au with that on Cu, it was found that the dissociative adsorption of O
Au with that on Cu, it was found that the dissociative adsorption of O is more activated (less reactive) due to Au alloying. The low-energy electron-diffraction (LEED) pattern of c(2
is more activated (less reactive) due to Au alloying. The low-energy electron-diffraction (LEED) pattern of c(2 2) for the clean surface turned into the (1
2) for the clean surface turned into the (1 1) LEED pattern during the oxidation by hyperthermal O
1) LEED pattern during the oxidation by hyperthermal O molecular beam, suggesting that the O adsorption induces the Cu-atom segregation on the surface.
molecular beam, suggesting that the O adsorption induces the Cu-atom segregation on the surface.
 on Si(111)-7
on Si(111)-7 7 surface at room temperature
7 surface at room temperatureYoshigoe, Akitaka; Naruhiro, Eisuke; Moritani, Kosuke; Teraoka, Yuden
Hyomen Kagaku, 27(8), p.449 - 454, 2006/08
Initial adsorption dynamics of O on Si(111)-7
on Si(111)-7 7 surface at room temperature was investigated by a supersonic molecular beam technique in conjunction with real-time X-ray photoelectron spectroscopy using synchrotron radiation. The initial sticking probability and the saturation coverage as a function of the translational kinetic energy of impinging O
7 surface at room temperature was investigated by a supersonic molecular beam technique in conjunction with real-time X-ray photoelectron spectroscopy using synchrotron radiation. The initial sticking probability and the saturation coverage as a function of the translational kinetic energy of impinging O were measured in the wide range from 0.03eV, namely thermal gas condition, to 2.3eV. We proposed a trapping-mediated process as a dominant adsorption mechanism at low kinetic energy region, while at high kinetic energies over 0.07eV the direct adsorption overlapping the trapping-mediated one whose contribution became small with increasing incident energies were expected to be a dominant adsorption mechanism. A gradual increase of the saturation coverage with increasing kinetic energy was clearly observed in the incident energy of 0.4eV to 1.7eV where the direct adsorption took over. The peak area intensity over 1.7eV regions is approximately 1.8 times larger than that for 0.03eV. These results indicate that the incident energy essentially induces the activated adsorption corresponding to the enhancement of the further O
were measured in the wide range from 0.03eV, namely thermal gas condition, to 2.3eV. We proposed a trapping-mediated process as a dominant adsorption mechanism at low kinetic energy region, while at high kinetic energies over 0.07eV the direct adsorption overlapping the trapping-mediated one whose contribution became small with increasing incident energies were expected to be a dominant adsorption mechanism. A gradual increase of the saturation coverage with increasing kinetic energy was clearly observed in the incident energy of 0.4eV to 1.7eV where the direct adsorption took over. The peak area intensity over 1.7eV regions is approximately 1.8 times larger than that for 0.03eV. These results indicate that the incident energy essentially induces the activated adsorption corresponding to the enhancement of the further O dissociative adsorption even at room temperature.
dissociative adsorption even at room temperature.
Takahashi, Shin*; Fujimoto, Yosuke*; Teraoka, Yuden; Yoshigoe, Akitaka; Moritani, Kosuke*; Aruga, Tetsuya*
KEK Proceedings 2006-3, p.80 - 82, 2006/08
Growth of oxygen adlayer on a Ru(0001) surface with high energy supersonic oxygen molecular beam was studied using high energy resolution X-ray photoelectron spectroscopy. The Ru 3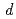
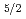 core level was used to monitor the growth of oxygen adlayer in the coverage region from 0.5 to 0.6 monolayer (ML) and the photoelectron spectrum was resolved into bulk and two surface components denoted as S1(2O) and S1(3O), which were first layer Ru atoms bonded to two and three oxygen atoms, respectively. The intensity change of each component as a function of oxygen dosage suggests the possibility of partial island formation on Ru(0001).
core level was used to monitor the growth of oxygen adlayer in the coverage region from 0.5 to 0.6 monolayer (ML) and the photoelectron spectrum was resolved into bulk and two surface components denoted as S1(2O) and S1(3O), which were first layer Ru atoms bonded to two and three oxygen atoms, respectively. The intensity change of each component as a function of oxygen dosage suggests the possibility of partial island formation on Ru(0001).
Moritani, Kosuke; Okada, Michio*; Fukuyama, Tetsuya*; Teraoka, Yuden; Yoshigoe, Akitaka; Kasai, Toshio*
European Physical Journal D, 38(1), p.111 - 115, 2006/04
Times Cited Count:14 Percentile:53.56(Optics)We report a study on the oxidation process induced by a hyperthermal oxygen molecular beam (HOMB) on Cu(110) using X-ray photoemission spectroscopy in conjunction with a synchrotron radiation source. The oxidation process induced by energetic O beams on Cu(110), depending on the azimuthal angle of incidence, suggests that the -Cu-O- added row structure has a role in inhibiting adsorption as a steric obstacle for incident O
beams on Cu(110), depending on the azimuthal angle of incidence, suggests that the -Cu-O- added row structure has a role in inhibiting adsorption as a steric obstacle for incident O molecules.
molecules.