Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 radical studied by picosecond pulse radiolysis
radical studied by picosecond pulse radiolysisEl Omar, A. K.*; Schmidhammer, U.*; Jeunesse, P.*; Labre, J. P.*; Lin, M.; Muroya, Yusa*; Katsumura, Yosuke*; Pernot, P.*; Mostafavi, M.*
Journal of Physical Chemistry A, 115(44), p.12212 - 12216, 2011/10
Times Cited Count:40 Percentile:81(Chemistry, Physical)Picosecond pulse radiolysis measurements using a pulse-probe method are performed to measure directly the time-dependent radiolytic yield of the OH radical in pure water. The time-dependent absorbance of OH
radical in pure water. The time-dependent absorbance of OH radical at 263 nm is deduced from the observed signal by substracting the contribution of the hydated electron and that of the irradiated empty fused silica cell which presents also a transient absoption. The time-dependent radiolytic yield of OH
radical at 263 nm is deduced from the observed signal by substracting the contribution of the hydated electron and that of the irradiated empty fused silica cell which presents also a transient absoption. The time-dependent radiolytic yield of OH is obtained by assuming the yield of the hydrated electron at 20 ps equal to 4.2
is obtained by assuming the yield of the hydrated electron at 20 ps equal to 4.2 10
10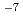 mol/J. The value of the yield of OH
mol/J. The value of the yield of OH radical at 20 ps is found to be (4.80
radical at 20 ps is found to be (4.80 0.12)
0.12) 10
10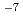 mol/J.
mol/J.
 , Br
, Br
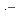 , and Br
, and Br
 in aqueous solutions
in aqueous solutionsLin, M.; Archirel, P.*; Van-Oanh, N. T.*; Muroya, Yusa*; Fu, H.*; Yan, Y.*; Nagaishi, Ryuji; Kumagai, Yuta; Katsumura, Yosuke*; Mostafavi, M.*
Journal of Physical Chemistry A, 115(17), p.4241 - 4247, 2011/04
Times Cited Count:14 Percentile:44.71(Chemistry, Physical)The absorption spectra of Br
 and Br
and Br
 in aqueous solutions are investigated by pulse radiolysis techniques from room temperature to 380 and 350
in aqueous solutions are investigated by pulse radiolysis techniques from room temperature to 380 and 350 C, respectively. The weak temperature effect on the absorption spectra of Br
C, respectively. The weak temperature effect on the absorption spectra of Br
 and Br
and Br
 is because in these two systems, the transition occurs between two valence states. We performed classical dynamics of hydrated Br
is because in these two systems, the transition occurs between two valence states. We performed classical dynamics of hydrated Br system at 20 and 300
system at 20 and 300 C under pressure of 25 MPa. This shows that the first water shell is strongly bound to the anion whatever the temperature. The first two water shells form a cavity of a roughly spherical shape around the anion. By TDDFT method, we calculated the absorption spectra of hydrated Br
C under pressure of 25 MPa. This shows that the first water shell is strongly bound to the anion whatever the temperature. The first two water shells form a cavity of a roughly spherical shape around the anion. By TDDFT method, we calculated the absorption spectra of hydrated Br at two temperatures and we compared the results with the experimental data.
at two temperatures and we compared the results with the experimental data.
Belloni, J.*; Crowell, R. A.*; Katsumura, Yosuke; Lin, M.; Marignier, J. L.*; Mostafavi, M.*; Muroya, Yusa*; Saeki, Akinori*; Tagawa, Seiichi*; Yoshida, Yoichi*; et al.
Recent Trends in Radiation Chemistry, p.121 - 160, 2010/05
In this review, we summarized development and application of highly time resolved spectroscopy system in order to measure radiation induced fast phenomena together with current status of the systems in the world.
Muroya, Yusa*; Lin, M.; De Waele, V.*; Hatano, Yoshihiko; Katsumura, Yosuke; Mostafavi, M.*
Journal of Physical Chemistry Letters (Internet), 1(1), p.331 - 335, 2010/01
Times Cited Count:43 Percentile:81.62(Chemistry, Physical)For the first time, a set-up is performed to carry out picoseconds time-resolved experiments in Supercritical Water (SCW) by pulse radiolysis. The kinetics of the decay of the hydrated electron is measured by transient absorption in the near IR on a time-scale ranging from 50 ps to 6 ns. The decay of the hydrated electrons in supercritical conditions shows a fast component at a short time below 500 ps, depending on the density of SCW. The results show that in SCW the main reaction for the decay of hydrated electron is that with H O
O .
.
Lin, M.; Fu, H.*; Lampre, I.*; De Waele, V.*; Muroya, Yusa*; Yan, Y.*; Yamashita, Shinichi; Katsumura, Yosuke; Mostafavi, M.*
Journal of Physical Chemistry A, 113(44), p.12193 - 12198, 2009/10
Times Cited Count:7 Percentile:22.44(Chemistry, Physical)With a revisit of the absorption coefficient of the solvated electron in propane-1,2,3-triol, the temperature dependent behavior of the absorption spectrum of solvated electron was studied from room temperature to 573 K by pulse radiolysis techniques. The change in the absorption spectrum of solvated electron in propane-1,2,3-triol observed by cooling down from a high temperature to 333 K is compared with that occurring during the electron solvation process at 333 K. The effect of the specific molecular structure of propane-1,2,3-triol compared to other alcohols is discussed.
Kumagai, Yuta; Lin, M.; Lampre, I.*; Mostafavi, M.*; Muroya, Yusa*; Katsumura, Yosuke
Radiation Physics and Chemistry, 77(10-12), p.1198 - 1202, 2008/10
Times Cited Count:5 Percentile:35.07(Chemistry, Physical)The absorption spectra of the hydrated electron in concentrated LiCl, LiClO , Li
, Li SO
SO , MgCl
, MgCl and Mg(ClO
and Mg(ClO )
) deuterated water solutions were measured by pulse radiolysis techniques from room temperature to 300
deuterated water solutions were measured by pulse radiolysis techniques from room temperature to 300  C at a constant pressure of 25 MPa. As salt effects, shifts to shorter wavelengths and broadening of the absorption band of the solvated electron were observed at room temperature. At elevated temperatures, similar spectral shifts were observed. The broadening of the spectrum, on the other hand, becomes smaller with increasing temperature. These result indicate that high concentration of salts not only form an ion atmosphere to cause the spectral shifts, but also affect the solvation structure of an electron. The smaller broadening at elevated temperatures suggest that a decrease in the density expand the distance between solvated electron and surrounding ions, and that therefore the effect on the solvation structure becomes smaller.
C at a constant pressure of 25 MPa. As salt effects, shifts to shorter wavelengths and broadening of the absorption band of the solvated electron were observed at room temperature. At elevated temperatures, similar spectral shifts were observed. The broadening of the spectrum, on the other hand, becomes smaller with increasing temperature. These result indicate that high concentration of salts not only form an ion atmosphere to cause the spectral shifts, but also affect the solvation structure of an electron. The smaller broadening at elevated temperatures suggest that a decrease in the density expand the distance between solvated electron and surrounding ions, and that therefore the effect on the solvation structure becomes smaller.
Lin, M.; Kumagai, Yuta; Lampre, I.*; Coudert, F.*; Muroya, Yusa*; Boutin, A.*; Mostafavi, M.*; Katsumura, Yosuke
Journal of Physical Chemistry A, 111(18), p.3548 - 3553, 2007/05
Times Cited Count:9 Percentile:30.94(Chemistry, Physical)Lin, M.; Mostafavi, M.*; Muroya, Yusa*; Lampre, I.*; Katsumura, Yosuke
Nuclear Science and Techniques, 18(1), p.2 - 9, 2007/02
Times Cited Count:1 Percentile:14.18(Nuclear Science & Technology)Lin, M.; Kumagai, Yuta; Muroya, Yusa*; Katsumura, Yosuke; Lampre, I.*; Coudert, F.*; Boutin, A.*; Mostafavi, M.*
no journal, ,
 s
sMuroya, Yusa*; Lin, M.; Han, Z.*; Kumagai, Yuta; Lampre, I.*; Mostafavi, M.*; Katsumura, Yosuke
no journal, ,
no abstracts in English
Lin, M.; Kumagai, Yuta; Muroya, Yusa*; Katsumura, Yosuke; Lampre, I.*; Coudert, F.*; Boutin, A.*; Mostafavi, M.*
no journal, ,
In this work, we measure the absorption spectra of hydrated electron in D O solutions containing different concentrations of Li
O solutions containing different concentrations of Li and Mg
and Mg cations at various temperatures by pulse radiolysis techniques, and perform quantum classical molecular dynamics (QCMD) simulations to explain the shift of the absorption spectra due to the combined effects of temperature and salt concentration.
cations at various temperatures by pulse radiolysis techniques, and perform quantum classical molecular dynamics (QCMD) simulations to explain the shift of the absorption spectra due to the combined effects of temperature and salt concentration.
Muroya, Yusa*; Lin, M.; Han, Z.*; Kumagai, Yuta; Lampre, I.*; Mostafavi, M.*; Katsumura, Yosuke
no journal, ,
In this work, we have studied the yield of solvated electron in ethylene glycol, 1,2-propanediol and 1,3-propanediol by electron beam radiation. Their time dependent behaviors in picosecond to microsecond range were also studied by a combination of picosecond and nanosecond pulse radiolysis techniques.
Lin, M.; Katsumura, Yosuke; Muroya, Yusa*; Fu, H.*; Yamashita, Shinichi; Mostafavi, M.*; Lampre, I.*
no journal, ,
The molar extinction coefficients at the absorption maximum of the solvated electron spectrum have been re-evaluated to be 9 000, 9 700, 10 000 and 10 600 M cm
cm for 1,2-ethanediol (12ED), 1,2-propanediol (12PD), and 1,3-propanediol (13PD) and glycerol (GLY), respectively. These values are two-thirds or three-fourths of the value usually reported in the published report. With these extinction coefficients, the time-dependent radiolytic yields of the solvated electron in these diols from the ps to the
for 1,2-ethanediol (12ED), 1,2-propanediol (12PD), and 1,3-propanediol (13PD) and glycerol (GLY), respectively. These values are two-thirds or three-fourths of the value usually reported in the published report. With these extinction coefficients, the time-dependent radiolytic yields of the solvated electron in these diols from the ps to the  s time range are described. The radiolytic yield in these viscous solvents is found to be strongly different from that of the aqueous solution. The temperature dependent absorption spectra of the solvated electron in 12ED, 12PD, 13PD, and GLY have also been investigated. In all these solvents, the optical spectra shift to the red with increasing temperature. The results, together with those of fs photochemistry studies carried out in Prof. Mostafavi's group, suggest that the solvation dynamics of electrons in these poly-ols is strongly related to the structure, especially the concentration of OH group.
s time range are described. The radiolytic yield in these viscous solvents is found to be strongly different from that of the aqueous solution. The temperature dependent absorption spectra of the solvated electron in 12ED, 12PD, 13PD, and GLY have also been investigated. In all these solvents, the optical spectra shift to the red with increasing temperature. The results, together with those of fs photochemistry studies carried out in Prof. Mostafavi's group, suggest that the solvation dynamics of electrons in these poly-ols is strongly related to the structure, especially the concentration of OH group.
Muroya, Yusa*; Lin, M.; Han, Z.*; Yamashita, Shinichi; Ueda, Toru*; Mostafavi, M.*; Katsumura, Yosuke
no journal, ,
A new ultrafast pulse radiolysis system was developed which enables to perform the experiments at high temperature/pressure condition covering supercritical condition. Fast spur decay kinetics as well as optical spectra of a hydrated electron in picosecond time scale at elevated temperature up to supercritical condition were observed for the first time.
Muroya, Yusa*; Lin, M.; Han, Z.*; Yamashita, Shinichi; Ueda, Toru*; Mostafavi, M.*; Katsumura, Yosuke
no journal, ,
By employing a newly developed high temperature - ultrafast pulse radiolysis system, temperature dependence of spur decay kinetics of the hydrated electron from room temperature up to supercritical condition was successfully measured. Faster spur decay within a few nanosecond was observed as increasing temperature. Picosecond yield of the hydrated electron at supercritical condition was also evaluated. The initial yield was found to show strongly density dependence of water.

 and Br
and Br
 in aqueous solutions
in aqueous solutionsLin, M.; Katsumura, Yosuke; Muroya, Yusa*; Fu, H.*; Yan, Y.*; Yamashita, Shinichi; Mostafavi, M.*
no journal, ,
The absorption spectra of Br
 and Br
and Br
 in aqueous solutions are investigated by pulse radiolysis techniques from room temperature to 380 and 350
in aqueous solutions are investigated by pulse radiolysis techniques from room temperature to 380 and 350  C, respectively. Temperature dependent spectrum of Br
C, respectively. Temperature dependent spectrum of Br is also measured under steady-sate. If the absorption of Br
is also measured under steady-sate. If the absorption of Br is of Charge Transfer To Solvent (CTTS), those of Br
is of Charge Transfer To Solvent (CTTS), those of Br
 and Br
and Br
 are not.
are not.
Muroya, Yusa*; Lin, M.; Han, Z.*; Yamashita, Shinichi; Ueda, Toru*; Hatano, Yoshihiko; Mostafavi, M.*; Katsumura, Yosuke
no journal, ,
By using a laser-driven S-band linear accelerator and a femtosecond laser, a new highly time-resolved pulse radiolysis system was developed for investigating radiation-induced fast phenomena in high temperature / high pressure water. Transient absorption spectra and fast kinetics of hydrated electron within several nanoseconds at elevated temperatures from room temperature up to supercritical state (400 C, 40 MPa) have been measured.
C, 40 MPa) have been measured.
Muroya, Yusa*; Lin, M.; Yan, Y.*; Han, Z.*; Yamashita, Shinichi; Ueda, Toru*; Mostafavi, M.*; Katsumura, Yosuke*
no journal, ,
no abstracts in English
Lin, M.; Katsumura, Yosuke*; Muroya, Yusa*; Yan, Y.*; Han, Z.*; Mostafavi, M.*; Lampre, I.*
no journal, ,
The temperature dependent absorption spectra of solvated electrons in some simple alcohols and four poly-alcohols, 1,2-ethanediol, 1,2-propanediol, 1,3-propanediol, and glycerol, have been systematically investigated by nanosecond pulse radiolysis techniques. The effects of molecular structure, especially the chain length and the number of OH group, on the absorption peak position are demonstrated. On the other hand, the temporal behaviours of solvated electron in methanol and the four poly-ols are studied by scavenging method and direct measurement using picosecond pulse radiolysis.
Lin, M.; Muroya, Yusa*; Katsumura, Yosuke*; Meesungneon, J.*; Jay-Gerin, J.-P.*; Mostafavi, M.*
no journal, ,
A setup was constructed to conduct picosecond time-resolved pulse radiolysis in UV region. Using this system, a direct observation of the picosecond kinetics of hydroxyl radical in pure H O, 2.0 M HClO
O, 2.0 M HClO aqueous solution and pure D
aqueous solution and pure D O was realized for the first time. A good agreement between the experimental results and Monte-Carlo simulations was demonstrated.
O was realized for the first time. A good agreement between the experimental results and Monte-Carlo simulations was demonstrated.