Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
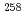 Md produced in the
Md produced in the 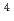 He +
He + 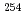 Es reaction
Es reactionNishio, Katsuhisa; Hirose, Kentaro; Makii, Hiroyuki; Orlandi, R.; Kean, K. R.*; Tsukada, Kazuaki; Toyoshima, Atsushi*; Asai, Masato; Sato, Tetsuya; Chiera, N. M.*; et al.
Physical Review C, 111(4), p.044609_1 - 044609_12, 2025/04
Times Cited Count:0 Percentile:0.00(Physics, Nuclear)Sato, Tetsuya; Nagame, Yuichiro*
Nihon Butsuri Gakkai-Shi, 78(2), p.64 - 72, 2023/02
The study of the chemistry of superheavy elements, which are located in the heavy extremes of the periodic table, has made considerable progress over the past 20 years, and new approaches based on various ideas have recently been developed. Research groups in Japan have also made significant contributions to the development of research on superheavy elements. Recently, notable results have been reported for the transactinide elements rutherfordium (element 104), dubnium (element 105), and seaborgium (element 106), and the heavy actinides with atomic numbers exceeding 100. The review will focus on the recent main results of these elements. This review outlines the main recent results and touches on future prospects.
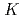 isomers in
isomers in 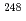 Cf
CfOrlandi, R.; Makii, Hiroyuki; Nishio, Katsuhisa; Hirose, Kentaro; Asai, Masato; Tsukada, Kazuaki; Sato, Tetsuya; Ito, Yuta; Suzaki, Fumi; Nagame, Yuichiro*; et al.
Physical Review C, 106(6), p.064301_1 - 064301_11, 2022/12
Times Cited Count:4 Percentile:52.14(Physics, Nuclear)Yakushev, A.*; Lens, L.*; D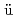 llmann, Ch. E.*; Khuyagbaatar, J.*; J
llmann, Ch. E.*; Khuyagbaatar, J.*; J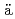 ger, E.*; Krier, J.*; Runke, J.*; Albers, H. M.*; Asai, Masato; Block, M.*; et al.
ger, E.*; Krier, J.*; Runke, J.*; Albers, H. M.*; Asai, Masato; Block, M.*; et al.
Frontiers in Chemistry (Internet), 10, p.976635_1 - 976635_11, 2022/08
Times Cited Count:19 Percentile:79.78(Chemistry, Multidisciplinary)Flerovium (Fl, element 114) is the heaviest element chemically studied so far. The first chemical experiment on Fl suggested that Fl is a noble-gas-like element, while the second studies suggested that Fl has a volatile-metal-like character. To obtain more reliable conclusion, we performed further experimental studies on Fl adsorption behavior on Si oxide and gold surfaces. The present results suggest that Fl is highly volatile and less reactive than the volatile metal, Hg, but has higher reactivity than the noble gas, Rn.
Sato, Tetsuya; Nagame, Yuichiro*
Radiochimica Acta, 110(6-9), p.441 - 451, 2022/07
Times Cited Count:1 Percentile:14.04(Chemistry, Inorganic & Nuclear)We describe our recent achievements in the effective production of low-energy ion-beams of the elements at the end of the actinide series, fermium (Fm, atomic number Z = 100), mendelevium (Md, Z = 101), nobelium (No, Z = 102), and lawrencium (Lr, Z = 103), using a surface ionization ion-source installed in the ISOL (Isotope Separator On-Line) at the Tandem accelerator facility of JAEA (Japan Atomic Energy Agency). Then the successful measurements of the first ionization potentials (IP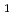 ) of these elements with the ISOL setup are reviewed. The measured IP
) of these elements with the ISOL setup are reviewed. The measured IP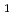 values increased up to No via Fm and Md, while that of Lr was the lowest among the actinides. Based on the variation of the IP1 values of the heavy actinides with the atomic number in comparison with those of the heavy lanthanides, the results clearly demonstrated that the 5f orbitals are fully filled at No, and the actinide series ends with Lr. Furthermore, the IP
values increased up to No via Fm and Md, while that of Lr was the lowest among the actinides. Based on the variation of the IP1 values of the heavy actinides with the atomic number in comparison with those of the heavy lanthanides, the results clearly demonstrated that the 5f orbitals are fully filled at No, and the actinide series ends with Lr. Furthermore, the IP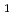 value of Lr provoked controversy over its position in the Periodic Table, so a short introduction to this issue is presented. The feasibility of the extension of chemical studies to still heavier elements with their ion-beams generated by ISOL is briefly discussed.
value of Lr provoked controversy over its position in the Periodic Table, so a short introduction to this issue is presented. The feasibility of the extension of chemical studies to still heavier elements with their ion-beams generated by ISOL is briefly discussed.
G tz, M.*; Yakushev, A.*; G
tz, M.*; Yakushev, A.*; G tz, S.*; Di Nitto, A.*; D
tz, S.*; Di Nitto, A.*; D llmann, Ch. E.*; Asai, Masato; Kindler, B.*; Krier, J.*; Lommel, B.*; Nagame, Yuichiro*; et al.
llmann, Ch. E.*; Asai, Masato; Kindler, B.*; Krier, J.*; Lommel, B.*; Nagame, Yuichiro*; et al.
Radiochimica Acta, 110(2), p.75 - 86, 2022/02
Times Cited Count:2 Percentile:20.20(Chemistry, Inorganic & Nuclear)The study of volatile superheavy element carbonyl complexes requires more efficient methods because the yield of transactinide elements decreases with increasing atomic number. This is achieved by using a newly developed double chamber system to separate the recoil chamber and the reaction one, thereby avoiding the decomposition of reactive molecules by the projectile ion beam, which hinders the synthesis of carbonyl complexes. The feasibility of this method was verified by synthesizing 5d metal short-lived isotopes as homologous element isotopes of the light transactinide elements Sg, Bh, Hs, and Mt at the Japan Atomic Energy Agency tandem accelerator and conducting model experiments.
Yakushev, A.*; Lens, L.*; D llmann, C. E.*; Block, M.*; Nagame, Yuichiro*; Sato, Tetsuya; Toyoshima, Atsushi*; 42 of others*
llmann, C. E.*; Block, M.*; Nagame, Yuichiro*; Sato, Tetsuya; Toyoshima, Atsushi*; 42 of others*
Frontiers in Chemistry (Internet), 9, p.753738_1 - 753738_9, 2021/11
Times Cited Count:19 Percentile:70.53(Chemistry, Multidisciplinary)Nihonium (Nh, element 113) and flerovium (Fl, element 114) are the first superheavy elements in which the 7p shell is occupied. High volatility and inertness were predicted for Fl due to the strong relativistic stabilization of the closed 7p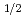 sub-shell, which originates from a large spin-orbit splitting between the 7p
sub-shell, which originates from a large spin-orbit splitting between the 7p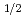 and 7p
and 7p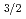 orbitals. One unpaired electron in the outermost 7p
orbitals. One unpaired electron in the outermost 7p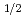 sub-shell in Nh is expected to give rise to a higher chemical reactivity. Theoretical predictions of Nh reactivity are discussed, along with results of the first experimental attempts to study Nh chemistry in the gas phase. The experimental observations verify a higher chemical reactivity of Nh atoms compared to its neighbor Fl and call for the development of advanced setups. First tests of a newly developed detection device miniCOMPACT with highly reactive Fr isotopes assure that effective chemical studies of Nh are within reach.
sub-shell in Nh is expected to give rise to a higher chemical reactivity. Theoretical predictions of Nh reactivity are discussed, along with results of the first experimental attempts to study Nh chemistry in the gas phase. The experimental observations verify a higher chemical reactivity of Nh atoms compared to its neighbor Fl and call for the development of advanced setups. First tests of a newly developed detection device miniCOMPACT with highly reactive Fr isotopes assure that effective chemical studies of Nh are within reach.

Chiera, N. M.*; Sato, Tetsuya; Eichler, R.*; Tomitsuka, Tomohiro; Asai, Masato; Adachi, Sadia*; Dressler, R.*; Hirose, Kentaro; Inoue, Hiroki*; Ito, Yuta; et al.
Angewandte Chemie; International Edition, 60(33), p.17871 - 17874, 2021/08
Times Cited Count:5 Percentile:24.90(Chemistry, Multidisciplinary)The formation and the chemical characterization of single atoms of dubnium (Db, element 105), in the form of its volatile oxychloride, was investigated using the on-line gas phase chromatography technique, in the temperature range 350 - 600 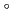 C. Under the exact same chemical conditions, comparative studies with the lighter homologs of group-5 in the Periodic Table clearly indicate the volatility sequence being NbOCl
C. Under the exact same chemical conditions, comparative studies with the lighter homologs of group-5 in the Periodic Table clearly indicate the volatility sequence being NbOCl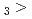 TaOCl
TaOCl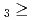 DbOCl
DbOCl . From the obtained experimental results, thermochemical data for DbOCl
. From the obtained experimental results, thermochemical data for DbOCl were derived. The present study delivers reliable experimental information for theoretical calculations on the chemical properties of transactinides.
were derived. The present study delivers reliable experimental information for theoretical calculations on the chemical properties of transactinides.
Nagame, Yuichiro*; Sato, Tetsuya; Kratz, J. V.*
Kirk-Othmer Encyclopedia of Chemical Technology (Internet), 52 Pages, 2020/09
This article gives a brief summary of the recent progress in the synthesis of new elements as well as heavy nuclei far from the stability line and in the studies of exotic nuclear decay properties including nuclear fission of heavy nuclei and chemical characterization of heavy actinides and transactinides. Experimental techniques of single-atom detection after in-flight separation with electromagnetic separators have made a breakthrough in discovery of new heavy isotopes. Development of automated rapid chemical separation apparatuses performing one atom-at-a-time chemistry has also considerably contributed to the progress of chemical studies of the transactinides. Some key experiments exploring new frontiers of the production and chemical characterization of heavy actinides and transactinides using state-of-the-art techniques are demonstrated. A short historical perspective of actinide and transactinide elements and some prospects of extending nuclear and chemical studies of heavy elements in the future are briefly presented.
Grund, J.*; Asai, Masato; Blaum, K.*; Block, M.*; Chenmarev, S.*; D llmann, Ch. E.*; Eberhardt, K.*; Lohse, S.*; Nagame, Yuichiro*; Nagy, Sz.*; et al.
llmann, Ch. E.*; Eberhardt, K.*; Lohse, S.*; Nagame, Yuichiro*; Nagy, Sz.*; et al.
Nuclear Instruments and Methods in Physics Research A, 972, p.164013_1 - 164013_8, 2020/08
Times Cited Count:8 Percentile:60.25(Instruments & Instrumentation)We report on the successful coupling of the Penning-trap mass spectrometry setup TRIGA-TRAP to the research reactor TRIGA Mainz. This offers the possibility to perform direct high-precision mass measurements of short-lived nuclei produced in neutron-induced fission of a 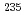 U target located near the reactor core. An aerosol-based gas-jet system is used for efficient transport of short-lived neutron-rich nuclei from the target chamber to a surface ion source. In conjunction with new ion optics and extended beam monitoring capabilities, the experimental setup has been fully commissioned. The design of the surface ion source, efficiency studies and first results are presented.
U target located near the reactor core. An aerosol-based gas-jet system is used for efficient transport of short-lived neutron-rich nuclei from the target chamber to a surface ion source. In conjunction with new ion optics and extended beam monitoring capabilities, the experimental setup has been fully commissioned. The design of the surface ion source, efficiency studies and first results are presented.
 -decay properties of
-decay properties of 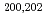 Fr
FrGhys, L.*; Andreyev, A. N.; Huyse, M.*; Van Duppen, P.*; Antalic, S.*; Barzakh, A.*; Capponi, L.*; Cocolios, T. E.*; Cubiss, J.*; Derkx, X.*; et al.
Physical Review C, 100(5), p.054310_1 - 054310_13, 2019/11
Times Cited Count:14 Percentile:75.29(Physics, Nuclear)Nagame, Yuichiro
Radiochimica Acta, 107(9-11), p.803 - 819, 2019/09
Times Cited Count:1 Percentile:8.72(Chemistry, Inorganic & Nuclear)Sch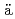 del, M.*; Nagame, Yuichiro
del, M.*; Nagame, Yuichiro
Radiochimica Acta, 107(7), p.561 - 585, 2019/07
Times Cited Count:4 Percentile:33.76(Chemistry, Inorganic & Nuclear)Chiera, N. M.; Sato, Tetsuya; Tomitsuka, Tomohiro; Asai, Masato; Ito, Yuta; Shirai, Kaori*; Suzuki, Hayato; Tokoi, Katsuyuki; Toyoshima, Atsushi; Tsukada, Kazuaki; et al.
Journal of Radioanalytical and Nuclear Chemistry, 320(3), p.633 - 642, 2019/06
Times Cited Count:2 Percentile:17.25(Chemistry, Analytical)An isothermal gas-chromatographic (IGC) device has been developed and tested for on-line gas phase studies of volatile oxychlorides of short-lived group-5 transition metals. Radioisotopes of niobium and tantalum, produced in nuclear fusion evaporation reactions, are directly flushed into the IGC setup by an inert gas-jet. Oxychloride compounds are formed by the addition of SOCl and O
and O . Parameters influencing the formation and transport of NbOCl
. Parameters influencing the formation and transport of NbOCl and TaOCl
and TaOCl are investigated. For nuclides with half-lives (
are investigated. For nuclides with half-lives (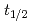 ) of about 30 s, an overall efficiency of 7% is obtained, rendering the IGC setup suitable for the chemical exploration of
) of about 30 s, an overall efficiency of 7% is obtained, rendering the IGC setup suitable for the chemical exploration of 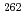 Db(
Db(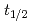 = 34s).
= 34s).
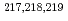 At revealed by in-source laser spectroscopy
At revealed by in-source laser spectroscopyBarzakh, A. E.*; Cubiss, J. G.*; Andreyev, A. N.; Seliverstov, M. D.*; Andel, B.*; Antalic, S.*; Ascher, P.*; Atanasov, D.*; Beck, D.*; Biero , J.*; et al.
, J.*; et al.
Physical Review C, 99(5), p.054317_1 - 054317_9, 2019/05
Times Cited Count:15 Percentile:73.32(Physics, Nuclear)Chiera, N. M.; Sato, Tetsuya; Tomitsuka, Tomohiro; Asai, Masato; Suzuki, Hayato*; Tokoi, Katsuyuki; Toyoshima, Atsushi; Tsukada, Kazuaki; Nagame, Yuichiro
Inorganica Chimica Acta, 486, p.361 - 366, 2019/02
Times Cited Count:4 Percentile:20.64(Chemistry, Inorganic & Nuclear)The formation of NbOCl and TaOCl
and TaOCl and their adsorption behavior on quartz surfaces was explored by applying an isothermal gas-chromatographic method. Trace amounts of short-lived Nb and Ta isotopes were used. Adsorption enthalpy values (
and their adsorption behavior on quartz surfaces was explored by applying an isothermal gas-chromatographic method. Trace amounts of short-lived Nb and Ta isotopes were used. Adsorption enthalpy values (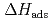 ) at zero surface coverage of -
) at zero surface coverage of -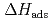 (NbOCl
(NbOCl ) = 102
) = 102  4 kJ/mol and -
4 kJ/mol and -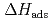 (TaOCl
(TaOCl ) = 128
) = 128  5 kJ/mol were determined by analyzing the chromatographic behavior of the Nb andTa complexes with a Monte-Carlo simulation method based on an adsorption-desorption kinetic model.By applying an empirical correlation, the experimental
5 kJ/mol were determined by analyzing the chromatographic behavior of the Nb andTa complexes with a Monte-Carlo simulation method based on an adsorption-desorption kinetic model.By applying an empirical correlation, the experimental 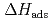 values were successively related to the macroscopic standard sublimation enthalpy,
values were successively related to the macroscopic standard sublimation enthalpy, 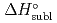 , as a measure of the volatility of each substance. The inferred sublimation enthalpies are in agreement with tabulated thermochemical values. Thus, the linear empirical correlation between
, as a measure of the volatility of each substance. The inferred sublimation enthalpies are in agreement with tabulated thermochemical values. Thus, the linear empirical correlation between 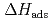 and
and 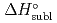 for metal-oxychlorides was updated with the inclusion of the present data. According to the predicted
for metal-oxychlorides was updated with the inclusion of the present data. According to the predicted 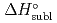 (DbOCl
(DbOCl ), a
), a 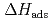 (DbOCl
(DbOCl ) value of 135
) value of 135  2 kJ/mol was extrapolated. The future accomplishment of comparative studies with DbOCl
2 kJ/mol was extrapolated. The future accomplishment of comparative studies with DbOCl under the same experimental conditions will provide valuable information on the volatility trend in Group-5 elements, together with an indication on the magnitude of relativistic effects on the electronic structure of dubnium.
under the same experimental conditions will provide valuable information on the volatility trend in Group-5 elements, together with an indication on the magnitude of relativistic effects on the electronic structure of dubnium.
 and Au surfaces in preparation for chemical investigations on Cn, Nh, and Fl at TASCA
and Au surfaces in preparation for chemical investigations on Cn, Nh, and Fl at TASCALens, L.*; Yakushev, A.*; D llmann, Ch. E.*; Asai, Masato; Ballof, J.*; Block, M.*; David, H. M.*; Despotopulos, J.*; Di Nitto, A.*; Eberhardt, K.*; et al.
llmann, Ch. E.*; Asai, Masato; Ballof, J.*; Block, M.*; David, H. M.*; Despotopulos, J.*; Di Nitto, A.*; Eberhardt, K.*; et al.
Radiochimica Acta, 106(12), p.949 - 962, 2018/12
Times Cited Count:11 Percentile:67.60(Chemistry, Inorganic & Nuclear)Online gas-solid adsorption studies with single atom quantities of Hg, Tl, and Pb on SiO and Au surfaces were carried out using short-lived radioisotopes with half-lives in the range of 4-49 s. This is a model study to measure adsorption enthalpies of superheavy elements Cn, Nh, and Fl. The short-lived isotopes were produced and separated by the gas-filled recoil separator TASCA at GSI. The products were stopped in He gas, and flushed into gas chromatography columns made of Si detectors whose surfaces were covered by SiO
and Au surfaces were carried out using short-lived radioisotopes with half-lives in the range of 4-49 s. This is a model study to measure adsorption enthalpies of superheavy elements Cn, Nh, and Fl. The short-lived isotopes were produced and separated by the gas-filled recoil separator TASCA at GSI. The products were stopped in He gas, and flushed into gas chromatography columns made of Si detectors whose surfaces were covered by SiO or Au. The short-lived Tl and Pb were successfully measured by the Si detectors with the SiO
or Au. The short-lived Tl and Pb were successfully measured by the Si detectors with the SiO surface at room temperature. On the other hand, the Hg did not adsorb on the SiO
surface at room temperature. On the other hand, the Hg did not adsorb on the SiO surface, but adsorbed on the Au surface. The results demonstrated that the adsorption properties of short-lived Hg, Tl, and Pb could be studied with this setup, and that this method is applicable to the experiment for Cn, Nh, and Fl.
surface, but adsorbed on the Au surface. The results demonstrated that the adsorption properties of short-lived Hg, Tl, and Pb could be studied with this setup, and that this method is applicable to the experiment for Cn, Nh, and Fl.
Sato, Tetsuya; Asai, Masato; Borschevsky, A.*; Beerwerth, R.*; Kaneya, Yusuke*; Makii, Hiroyuki; Mitsukai, Akina*; Nagame, Yuichiro; Osa, Akihiko; Toyoshima, Atsushi; et al.
Journal of the American Chemical Society, 140(44), p.14609 - 14613, 2018/11
Times Cited Count:32 Percentile:69.40(Chemistry, Multidisciplinary)The first ionization potential (IP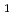 ) yields information on valence electronic structure of an atom. IP
) yields information on valence electronic structure of an atom. IP values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP
values of heavy actinides beyond einsteinium (Es, Z = 99), however, have not been determined experimentally so far due to the difficulty in obtaining these elements on scales of more than one atom at a time. Recently, we successfully measured IP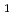 of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP
of lawrencium (Lr, Z = 103) using a surface ionization method. The result suggests that Lr has a loosely-bound electron in the outermost orbital. In contrast to Lr, nobelium (No, Z = 102) is expected to have the highest IP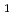 among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP
among the actinide elements owing to its full-filled 5f and the 7s orbitals. In the present study, we have successfully determined IP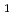 values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP
values of No as well as fermium (Fm, Z = 100) and mendelevium (Md, Z = 101) using the surface ionization method. The obtained results indicate that the IP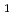 value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.
value of heavy actinoids would increase monotonically with filling electrons up in the 5f orbital like heavy lanthanoids.
Nagame, Yuichiro
Radioisotopes, 67(10), p.507 - 526, 2018/10
Recent studies of the chemical separation and characterization experiments of the first three transactinide elements, rutherfordium (Rf), dubnium (Db) and seaborgium (Sg), conducted atom-at-a-time in liquid phases, are reviewed. A short description on experimental techniques based on partition methods, specifically automated rapid chemical separation systems, as well as on assessment of role of relativistic effects is also given. Perspectives for liquid phase chemistry experiments on heavier elements are briefly discussed.
 SO
SO and HF/HCl solutions into toluene with Aliquat336; Sulfate and fluoride complex formation of Mo and W towards chemical studies of seaborgium (Sg)
and HF/HCl solutions into toluene with Aliquat336; Sulfate and fluoride complex formation of Mo and W towards chemical studies of seaborgium (Sg)Toyoshima, Atsushi; Mitsukai, Akina; Tsukada, Kazuaki; Oe, Kazuhiro*; Haba, Hiromitsu*; Komori, Yukiko*; Murakami, Masashi; Kaneya, Yusuke*; Sato, Daisuke*; Asai, Masato; et al.
Journal of Radioanalytical and Nuclear Chemistry, 317(1), p.421 - 430, 2018/07
Times Cited Count:3 Percentile:25.46(Chemistry, Analytical)We have studied extraction behavior of group-6 elements Mo and W to search for suitable conditions for an on-line extraction experiment of their heavier homolog, seaborgium (Sg). Batch-wise extraction of carrier-free radiotracers 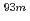 Mo and
Mo and 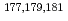 W were carried out from 0.10 - 8.6 M H
W were carried out from 0.10 - 8.6 M H SO
SO and 1.0
and 1.0 10
10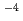 - 5.0 M HF/1.0 M HCl into toluene with a quaternary ammonium compound, Aliquat336. Anionic sulfate complexes of Mo and W with charge - 2 were extracted with Aliquat336 from H
- 5.0 M HF/1.0 M HCl into toluene with a quaternary ammonium compound, Aliquat336. Anionic sulfate complexes of Mo and W with charge - 2 were extracted with Aliquat336 from H SO
SO solutions with concentrations of [H
solutions with concentrations of [H SO
SO ]
]  2 M. In HF/1.0 M HCl, oxyfluoro complexes of Mo and W with charge - 1 were interpreted to be formed and extracted with Aliquat336. From these results, favorable conditions for the extraction of Sg are discussed.
2 M. In HF/1.0 M HCl, oxyfluoro complexes of Mo and W with charge - 1 were interpreted to be formed and extracted with Aliquat336. From these results, favorable conditions for the extraction of Sg are discussed.