Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hatakeyama, Mizuki*; Nishiyama, Yoshio*; Nagatani, Hirohisa*; Okamura, Hiroyuki; Imura, Hisanori*
Solvent Extraction Research and Development, Japan, 25(2), p.79 - 89, 2018/00
Times Cited Count:12 Percentile:42.69(Chemistry, Multidisciplinary)The synergistic extraction of trivalent lanthanide ions (Ln(III)) with benzoylacetone (Hba) and trioctyphosphine oxide (TOPO) in an ionic liquid (IL), 1-butyl-3-methyl-imidazolium bis(trifluoromethanesulfonyl)imide ([Bmim][Tf N]), has been investigated. The extractability of Ln(III) with Hba was significantly enhanced in the presence of TOPO. The composition and extraction constants of the extracted species for each Ln(III) were determined by 3-dimensional equilibrium analysis. It was found that all Ln(III) were extracted as cationic ternary complexes such as Ln(ba)
N]), has been investigated. The extractability of Ln(III) with Hba was significantly enhanced in the presence of TOPO. The composition and extraction constants of the extracted species for each Ln(III) were determined by 3-dimensional equilibrium analysis. It was found that all Ln(III) were extracted as cationic ternary complexes such as Ln(ba) (TOPO)
(TOPO)
 or Ln(ba)(TOPO)
or Ln(ba)(TOPO)
 with Hba and TOPO. Furthermore, the formation constants of the cationic ternary complexes in the IL indicate that Lu(ba)(TOPO)
with Hba and TOPO. Furthermore, the formation constants of the cationic ternary complexes in the IL indicate that Lu(ba)(TOPO)
 is the most stable complex in the IL.
is the most stable complex in the IL.
 -diketones and trioctylphosphine oxide in an ionic liquid
-diketones and trioctylphosphine oxide in an ionic liquidOkamura, Hiroyuki; Takagi, Hitomi*; Isomura, Taku*; Morita, Kotaro*; Nagatani, Hirohisa*; Imura, Hisanori*
Analytical Sciences, 30(3), p.323 - 325, 2014/03
Times Cited Count:31 Percentile:74.29(Chemistry, Analytical) -diketone and hydrophobic neutral ligand; Specific effect of
-diketone and hydrophobic neutral ligand; Specific effect of  -diketones
-diketonesImura, Hisanori*; Okamura, Hiroyuki; Takagi, Hitomi*; Isomura, Taku*; Hatakeyama, Mizuki*; Morita, Kotaro*; Nagatani, Hirohisa*
no journal, ,
no abstracts in English
 -diketones and a hydrophobic neutral ligand
-diketones and a hydrophobic neutral ligandOkamura, Hiroyuki; Takagi, Hitomi*; Isomura, Taku*; Hatakeyama, Mizuki*; Morita, Kotaro*; Nagatani, Hirohisa*; Imura, Hisanori*
no journal, ,
 -diketones on the extracted species
-diketones on the extracted speciesOkamura, Hiroyuki; Hatakeyama, Mizuki*; Nagatani, Hirohisa*; Nishiyama, Yoshio*; Shimojo, Kojiro; Naganawa, Hirochika; Imura, Hisanori*
no journal, ,
In the separation of lanthanoids (Lns), the synergistic extraction systems have been widely investigated to enhance the extractability and separability. Recently, a method of extraction equilibrium analysis for Ln(III) in the ionic liquid (IL) synergistic extraction system with 2-thenoyltrifluoroacetone (Htta)-trioctylphosphine oxide (TOPO) in 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide was reported. In this study, the extraction equilibrium of Ln in the benzoylacetone (Hba)-TOPO system exhibiting higher Ln separability than the Htta-TOPO system was investigated. The effect of  -diketones on the extracted species and the relationship between the extracted species and Ln separability were clarified. A three-dimensional non-linear analysis of log
-diketones on the extracted species and the relationship between the extracted species and Ln separability were clarified. A three-dimensional non-linear analysis of log  -log [ba
-log [ba ]
]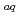 -log [TOPO]
-log [TOPO]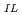 was applied in the Hba-TOPO synergistic extraction system, and both Ln(ba)
was applied in the Hba-TOPO synergistic extraction system, and both Ln(ba) (TOPO)
(TOPO)
 and Ln(ba)(TOPO)
and Ln(ba)(TOPO)
 were found as the extracted species. The formation of 1:1:4 dicationic complex was not observed in the Htta-TOPO synergistic extraction system, indicating that the extracted species is dependent on the acidity of
were found as the extracted species. The formation of 1:1:4 dicationic complex was not observed in the Htta-TOPO synergistic extraction system, indicating that the extracted species is dependent on the acidity of  -diketone. Consequently, it was suggested that the formation of the highly hydrophobic 1:1:4 complex is a factor of higher Ln separability.
-diketone. Consequently, it was suggested that the formation of the highly hydrophobic 1:1:4 complex is a factor of higher Ln separability.